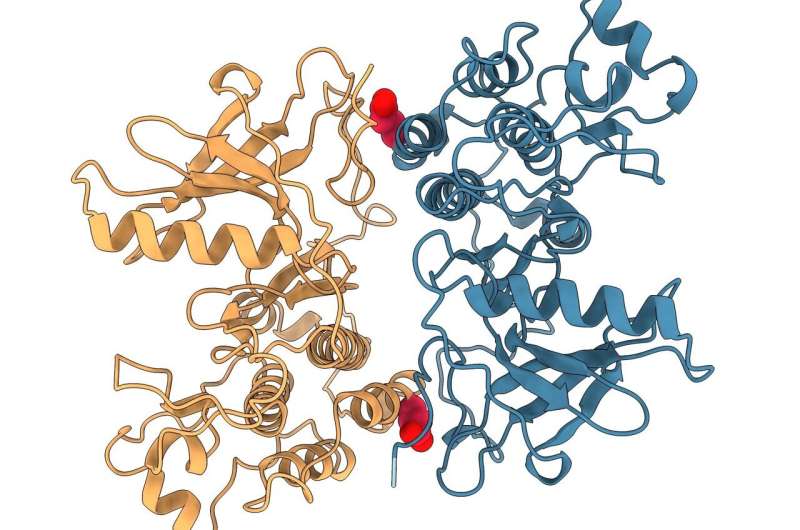
Rendering of interface in the mutant EGFR studied that was shown to be critical for EGFR-driven tumor growth. The K946E mutation shown in red was used by the team to confirm that this interface is implicated in tumor growth in the study. Credit: Ioannis Galgadas, University of Geneva
Researchers have shown for the first time that a crucial interface in a protein that drives cancer growth could act as a target for more effective treatments.
The study was led by the Science and Technology Facilities Council (STFC) Central Laser Facility (CLF) and used advanced laser imaging techniques to identify structural details of a mutated protein that help it to evade drugs that target it.
The research is published in the journal Nature Communications and lays the groundwork for future research into more effective, long-lasting cancer therapies.
The Epidermal Growth Factor Receptor (EGFR) is a protein that sits on the surface of cells and receives molecular signals that tell the cell to grow and divide. In certain types of cancer, mutated EGFR stimulates uncontrolled growth, resulting in tumors. Various cancer treatments block and inhibit mutant EGFR to prevent tumor formation, but these are limited, as eventually cancerous cells commonly develop further EGFR mutations that are resistant to treatment.
Until now, how exactly these drug-resistant EGFR mutations drive tumor growth has not been understood, hindering our ability to develop treatments that target them.
In this latest study, scientists at CLF have obtained super-resolution images of a drug-resistant EGFR mutation known to contribute to lung cancer. This was achieved using an advanced laser imaging technique developed by STFC for this purpose and called Fluorophore Localization Imaging with Photobleaching, or FLImP.
FLImP analysis revealed structural details as small as two nanometers and showed for the first time with this level of precision how molecules in the drug-resistant EGFR mutation interact.
Additional analysis by the Biomolecular & Pharmaceutical Modeling Group at University of Geneva (UNIGE) used advanced computer simulations that—combined with the FLImP analysis—provided atomistic details of the mutant EGFR complexes. From this, the team was able to compare the structural details of the mutated and healthy EGFR to identify interfaces between interacting molecules in the drug-resistant mutation critical for tumor growth.
Professor Marisa Martin-Fernandez, Leader of the Octopus Group at CLF, which led the study, said, “This finding is the culmination of years of research and technological development at CLF and our partner institutions and we’re extremely excited about its potential to inform the course of cancer research going forward. If this interface proves to be an effective therapeutic target, it could provide an entirely new approach to much needed pharmaceutical development.”
The team then introduced additional mutations to the drug-resistant EGFR in in cultured lung cells and in mice that interfered with the newly discovered interfaces.
In these experiments, one of the additional EGFR mutations was shown to block cancer growth, with mice developing no tumors, further indicating that the ability of this EGFR mutation to promote cancer indeed depends on these interfaces.
Dr. Gilbert Fruhwirth, Leader of the Imaging Therapies and Cancer group at King’s College London who validated results in live animals, remarked, “This research has become possible through the combination of a variety of different imaging technologies, ranging from single molecules to whole animals, and demonstrates the power of imaging to better understand the inner workings of cancer. We are extremely pleased about this successful collaboration and look forward to develop this pharmaceutical opportunity further as part of this team.”
Researchers hope that these interfaces could act as potential targets for new cancer therapies that overcome resistance acquired by EGFR mutations.
Professor Francesco Luigi Gervasio, Leader of the Biomolecular & Pharmaceutical Modeling Group at UNIGE, commented, “This breakthrough was made possible by a combination of state-of-the-art simulations and experimental techniques that can now ‘visualize’ the structure and dynamics of important cancer targets such as EGFR in unprecedented detail.”
Dr. Yiannis Galdadas at UNIGE, who performed the simulations, added, “The simulations were able to push the effective resolution of the microscope beyond the limits of imagination. It’s almost possible to ‘touch’ the mutation site and see its effect.”
Further studies at CLF are currently testing the research method on other EGFR mutations known to contribute to lung cancer. They also hope to establish whether this interface plays a role in the development of other cancers including brain cancer.
More information:
Drug-resistant EGFR mutations promote lung cancer by stabilizing interfaces in ligand-free kinase active EGFR oligomers, Nature Communications (2024). DOI: 10.1038/s41467-024-46284-x. www.nature.com/articles/s41467-024-46284-x
Citation:
Scientists identify Achilles heel of lung cancer protein (2024, March 19)
retrieved 19 March 2024
from https://medicalxpress.com/news/2024-03-scientists-achilles-heel-lung-cancer.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.
>>> Read full article>>>
Copyright for syndicated content belongs to the linked Source : Medical Xpress – https://medicalxpress.com/news/2024-03-scientists-achilles-heel-lung-cancer.html










