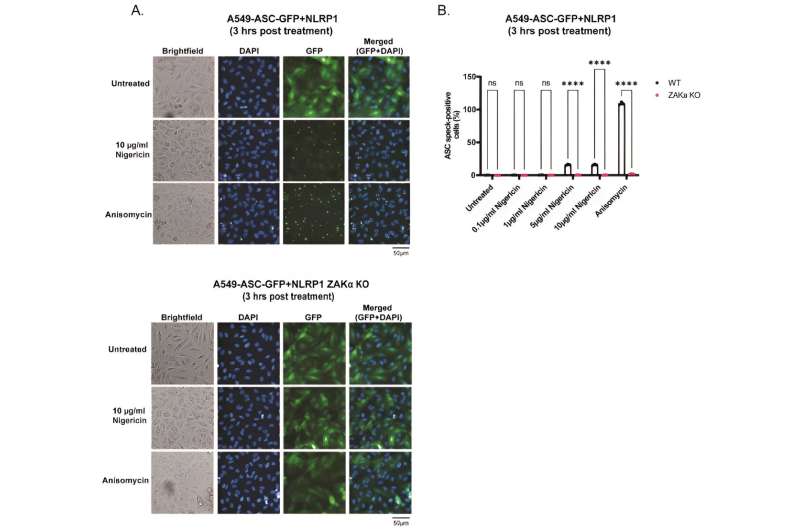
A. Representative images of A549-ASC-GFP-NLRP1 (upper panel) or ZAKɑ KO A549-ASCGFP-NLRP1 (lower panel) reporter cells Images of GFP and DAPI fluorescence were acquired 3 hours post treatment with nigericin (10 µg/ml) or anisomycin (1 µM) at 20x magnification. Images are shown from one experiment and are representative o f n=3 independent experiments; scale bar, 50 µm. B. ASC-speck quantification from images obtained in A. Credit: Proceedings of the National Academy of Sciences (2024). DOI: 10.1073/pnas.2309579121
Researchers co-led by Nanyang Technological University, Singapore (NTU Singapore) and the University of Toulouse, France, have uncovered how bacteria and their toxins prompt the human immune response, leading to inflammation.
Inflammation plays a crucial role in fighting infections and healing injuries, but when it is persistent, it can also contribute to adverse side effects in chronic diseases such as heart disease and diabetes.
Inflammation can also trigger autoimmune disorders such as lupus, when the immune system mistakenly attacks the body’s tissues, causing widespread inflammation and damage to organs like the joints, skin, brain, lungs, kidneys, and blood vessels.
Writing in the Proceedings of the National Academy of Sciences, the researchers report a direct link between the molecules that move ions in and out of cells, known as ionophores, the consequent change of the salt content within human cells, and inflammation.
In the study, the researchers found that when the level of potassium ions within cells falls below a certain level, the cells kickstart an immune response and release strong pro-inflammatory molecules, such as those which can lead to the sensation of pain and fever and contribute to the tissue damage caused by infections.
Past research has shown that a human gene known as NLRP3 is essential to control this process in the blood. Now, the NTU Singapore and University of Toulouse study demonstrates for the first time that this process is controlled by a pair of genes known as NLRP1 and ZAKα in human organs such as the skin, lungs, and nose.
Lead author Assistant Professor Franklin Zhong from NTU’s Lee Kong Chian School of Medicine (LKCMedicine), said, “Cells use a lot of energy to move sodium and potassium ions across their membranes, maintaining a specific ion balance between the interior of the cells and the external environment. This balance is crucial for normal cell functions. Problems with this balance can lead to diseases like neurological disorders and heart failure.
“Our study demonstrates that the human innate immune system has evolved multiple ways to sense disruptions of the cellular ion balance. This discovery helps us see how cells defend themselves when the balance of ions goes haywire, particularly when under pathogen attack. Our findings present a new piece in the puzzle of how our immune system functions and it could open doors to better treatments for diseases such as severe bacterial or viral infections.”
Co-lead author Dr. Etienne Meunier, Group Leader at the Institute of Pharmacology and Structural Biology at the University of Toulouse, France, said, “As the basic unit of life, cells need to carefully monitor for any alteration of their function, which includes those induced upon threats of infectious origin, but also daily perturbations that could occur in the body. Here, the findings that several of our cells express a defensive pathway that can detect and respond to defects in several essential ions for the life of cells constitute a novel fundamental knowledge important in our understanding of the way our body works.”
Providing an independent comment on the study, Professor Joseph Sung, NTU Senior Vice President (Health & Life Sciences) and Dean of NTU’s Lee Kong Chian School of Medicine, said, “We have learned from the severe acute respiratory syndrome (SARS) and COVID-19 pandemics is that an over-reacting immune system can harm our body, instead of protecting us. Understanding the mechanism of harnessing immune responses when it comes to infections will certainly help to prevent post-infection injuries.”
The study, which represents an advance in understanding how our bodies respond to infections, reflects NTU’s commitment to responding to the needs and challenges of healthy living and aging, one of humanity’s grand challenges that the University seeks to address through its NTU 2025 strategic plan.
Authors contributing to this study include first authors LKCMedicine Ph.D. student Pritisha Rozario, Miriam Pinilla, from the University of Toulouse, and contributing authors Prof Simon Bekker-Jensen from the University of Copenhagen, and Prof John Chambers, Director of Health Screening Center at NTU LKCMedicine.
Decoding cellular alarms: How cells speak the language of immune defense
To arrive at their results, the researchers grew healthy cells from the human skin and airway in a lab dish and exposed them to an ionophore known as nigericin, that is also known as an antibiotic.
After this, they analyzed the cells for swelling in their cell membranes, measuring the ion concentrations within the cells, and testing for the presence of inflammatory molecules, Gasdermin D and interleukin-1, released from the cells. These molecules are telltale signs that the human immune system has responded to infection or cellular damage by programming cells to self-destruct.
They also trigger an inflammatory cascade, a process equivalent to setting off an alarm call for help, which brings in immune cells and causes inflammation to fight off the threat and leads to common inflammatory symptoms such as swelling and fever.
Asst Prof Zhong added, “Our research has significant implications, especially in the context of antibiotic-resistant bacteria. Antibiotic resistance is a growing concern, and traditional antibiotics may become less effective in treating certain infections.
“In many cases, the morbidity of these infections is caused by both direct effects of the bacteria and the dysregulated inflammatory response that misfires against the host organs. Identifying alternative ways to modulate immune responses, such as targeting NLRP1 and NLRP3, could pave the way for innovative approaches to tackle infections.”
Dr. Meunier added, “Beyond the sole understanding of how our immunity works, unraveling specific mechanisms engaged by our cells in various contexts allows identifying novel cellular effectors that could serve as future pharmacological targets in a broader range of diseases than anticipated.”
The research team will continue their research to delve deeper into our immune responses, exploring how these signals might pave the way for tailored therapies that bolster the body’s capacity to combat infections with precision.
They hope the ongoing research will contribute to developing more effective treatments for infectious diseases.
More information:
Pritisha Rozario et al, Mechanistic basis for potassium efflux–driven activation of the human NLRP1 inflammasome, Proceedings of the National Academy of Sciences (2024). DOI: 10.1073/pnas.2309579121
Citation:
How the body’s immune response to bacterial infections could cause detrimental inflammation (2024, March 20)
retrieved 20 March 2024
from https://medicalxpress.com/news/2024-03-body-immune-response-bacterial-infections.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.
>>> Read full article>>>
Copyright for syndicated content belongs to the linked Source : Medical Xpress – https://medicalxpress.com/news/2024-03-body-immune-response-bacterial-infections.html










