Multitarget stool-based tests are showing promise for colorectal cancer (CRC) screening in average-risk individuals and could edge out the current standard fecal immunochemical test (FIT).
These new tests aren’t radical departures from the standard FIT. Like the standard test, the multitarget FIT uses antibodies to test for hemoglobin in stool samples. But these multitarget approaches take the standard FIT a step further by testing for additional DNA, RNA, or protein biomarkers associated with CRC to help improve early detection.
Currently, the US Preventive Services Task Force (USPSTF) recommends two FIT tests — standard FIT and stool FIT-DNA — as well as a third noninvasive CRC screening test, guaiac fecal occult blood test (gFOBT). gFOBT detects heme, a component of hemoglobin, through a chemical reaction.
But both standard FIT and stool FIT-DNA come with caveats. Compared to the standard test, FIT-DNA tends to be better at detecting traces of blood in the stool, and thus can uncover more instances of CRC or other advanced lesions. The flipside is that the DNA test also often leads to more false-positive findings.
In fact, the American College of Physicians does not recommend stool FIT-DNA for screening, citing issues such as cost — more than $600 per test vs about $30 for standard FIT — and the greater likelihood of false-positives compared with both standard FIT and gFOBT.
Given these tradeoffs with current noninvasive screening options, developing a FIT option that can improve early detection of CRC and advanced precancerous lesions without increasing false-positives could make a big difference in outcomes.
Three new noninvasive multitarget tests under investigation — an updated DNA-based test, Cologuard 2.0 (Exact Sciences; Madison, WI); an RNA-based test, ColoSense (Geneoscopy; St Louis, MO); and a protein-based test from CRCbioscreen (Amsterdam, the Netherlands) — may be able to do just that.
Cologuard 2.0: Multitarget Stool DNA-Based Test
An updated version of the stool FIT-DNA is currently under development. Dubbed Next Generation Cologuard, or Cologuard 2.0, this multitarget test detects three novel methylated DNA markers along with fecal hemoglobin.
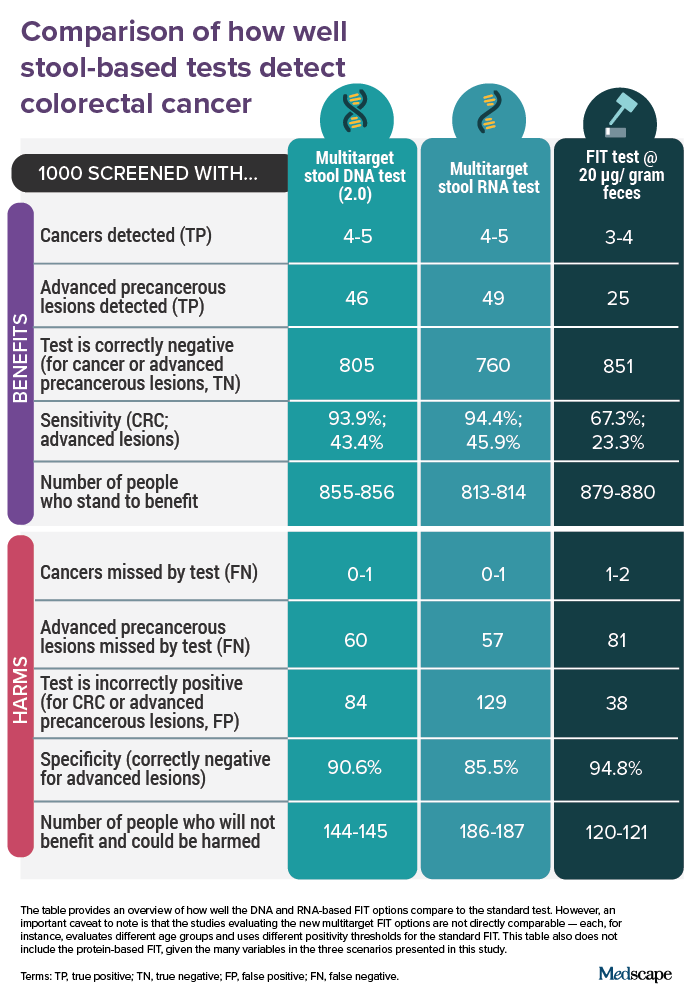
In a recent trial comparing Cologuard 2.0 vs standard FIT, 20,176 participants aged 40 years or older were screened with Cologuard 2.0 as well as standard FIT before they all also received a colonoscopy. The researchers compared findings with Cologuard 2.0 and standard FIT, which used a positivity cutoff ≥ 20 µg hemoglobin/g feces.
The researchers then assessed Cologuard 2.0’s sensitivity (a gauge of how well it detects disease that is truly present) and specificity (a measure of how well a test indicates the absence of disease when no disease is present) compared with standard FIT and the original Cologuard test.
Overall, Cologuard 2.0 demonstrated better sensitivity for CRC than did standard FIT (93.9% vs 67.3%, respectively) and for advanced precancerous lesions (43.4% vs 23.3%). The next-generation test, for instance, identified 92 of 98 participants with colonoscopy-confirmed CRC diagnoses vs 66 cases using standard FIT.
Compared with the original Cologuard, Cologuard 2.0’s sensitivity improved slightly for CRC, from 92% to 93.9%,; for advanced precancerous lesions, from 42% to 43.4%; and for high-grade dysplasia, from 69% to 75%. Specificity also improved with the latest version, from 87% to 90.6%.
However, Cologuard 2.0’s specificity for advanced neoplasia was worse than that of standard FIT (90.6% vs 94.8%, respectively), which would increase the likelihood of false-positive findings.
Despite its lower specificity compared with standard FIT, Cologuard 2.0 has several advantages. The test can identify more people with CRC and advanced precancerous lesions than the standard test and can lead to fewer false-positives than the original Cologuard test.
Cologuard maker Exact Sciences has submitted trial data to the US Food and Drug Administration (FDA) for approval.
Multitarget Stool RNA-Based Test
ColoSense, an RNA-based stool test, looks for eight RNA biomarkers associated with CRC.
The company says that RNA-based testing has an advantage over DNA biomarker assays, such as the currently marketed Cologuard test, because it isn’t subject to the age-related changes in DNA methylation that can throw off the results from DNA assays.
Like Cologuard 2.0, Geneoscopy’s Colosense test is under review by the FDA.
The data Geneoscopy submitted to the FDA came from the CRC-PREVENT trial, which included 8920 participants who were screened with both ColoSense and standard FIT before all had a colonoscopy. The participants ranged in age from 45 to 90 years, with 22% between 45 and 50 years old, a population recently added to the USPSTF screening recommendations.
ColoSense showed higher sensitivity than standard FIT for the presence of CRC (94% vs 78%, respectively) and advanced adenomas (46% vs 29%). In the group aged 45-50 years, the RNA-based test had a sensitivity of 100% for CRC, correctly identifying all five people with colonoscopy-confirmed CRC, and 45% for advanced adenomas.
However, ColoSense was less specific than standard FIT compared with negative colonoscopy findings (88% vs 96%, respectively) and negative findings for advanced lesions or CRC (85.5% vs 94.9%); thus, it was more likely to lead to false-positive results.
Overall, the investigators said ColoSense is comparable to Cologuard — its chief market rival — in terms of sensitivity for CRC and advanced adenomas but has higher sensitivity for colorectal neoplasia in people aged 50 years or younger.
Multitarget Protein-Based Test
The multitarget protein-based FIT uses antibodies to test for two additional proteins: calprotectin, an inflammatory marker associated with CRC, and serpin family F member 2, a protease inhibitor thought to be upregulated in colon cancer.
A 2021 study of 1284 patients found that the sensitivity of the multitarget protein-based test was 42.9% for advanced neoplasias compared with 37.3% with standard FIT. Its specificity was similar to that of standard FIT, at 96.6% for advanced neoplasias.
In a more recent report published in The Lancet Oncology, the team modeled three scenarios comparing the two FIT tests. These scenarios used different cutoff values for a test to be positive for CRC or an advanced lesion.
Overall, the analysis included stool samples from 13,187 patients aged 55-75 years who were in the Netherlands’ national CRC screening program. Stool samples were evaluated with both the multitarget test and the standard FIT, using a positivity cutoff ≥ 47 µg hemoglobin/g feces. Colonoscopy data were available for only 1270 participants.
In scenario 1, the multitarget test had a lower threshold for a positive test and consequently identified more precancerous lesions than the standard FIT (828 vs 354, respectively). The multitarget FIT identified a few more CRC cases: Of 29 colonoscopy-confirmed CRC cases, the multitarget FIT identified 26 vs 23 with standard FIT.
But the multitarget FIT also had more than double the number of false-positives than the standard FIT (347 vs 161, respectively).
Perhaps the most telling comparison occurred in scenario 2, with both tests set at the same low positivity threshold to minimize false-positives.
As expected, the two tests had similar positivity rates for advanced lesions, with the multitarget test correctly identifying 22 of 29 people with CRC, one fewer than the standard test. The protein-based test identified slightly more people with advanced lesions (156 vs 136 with the standard test), leading to a higher sensitivity for advanced lesions.
Most notably, the protein-based test resulted in fewer false-positives than did the standard test (295 vs 311, respectively) , resulting in a slightly higher specificity.
In this scenario, “a single screening round might not have the biggest impact on cancer incidence and mortality,” the authors said, but the higher detection rate would still accumulate over 20 years of testing. The authors estimated that, under this scenario, substituting the multitarget FIT for the standard test in the Netherlands’ CRC screening program could reduce CRC incidence by 5% and CRC mortality by 4%.
Gerrit Meijer, MD, PhD, a pathologist at the Netherlands Cancer Institute, and colleagues recently launched a company called CRCbioscreen to commercialize this multitarget FIT for large-scale programs. The company’s priority is to develop and validate a clinical-grade test to sell to federal governments with national screening programs, such as those throughout Europe, Australia, and Asia, Meijer told Medscape Medical News. Meijer expects this process will take about 4 years.
The test will be developed for the US market, but with no nationwide screening program in the United States, future availability will depend on interest from providers and institutions, noted Meijer, who is also chief scientific officer at CRCbioscreen.
Overall, these three new multitarget stool-based CRC screening tests could help catch more cancers and advanced precancerous lesions. And, if the tests have a high enough specificity, a negative test result could also allow people to forgo screening colonoscopy.
Still, people with a positive FIT finding would require follow-up colonoscopy, but about 10% of patients decline colonoscopy following an abnormal FIT, Mark A. Lewis, MD, director of gastrointestinal oncology at Intermountain Health in Murray, Utah, told Medscape Medical News last year. That means that even if precancerous lesions and CRC are being caught earlier, treatment can’t be started unless people follow through with colonoscopy.
>>> Read full article>>>
Copyright for syndicated content belongs to the linked Source : Medscape – https://www.medscape.com/viewarticle/what-know-about-next-gen-fit-crc-screening-2024a10006yo










