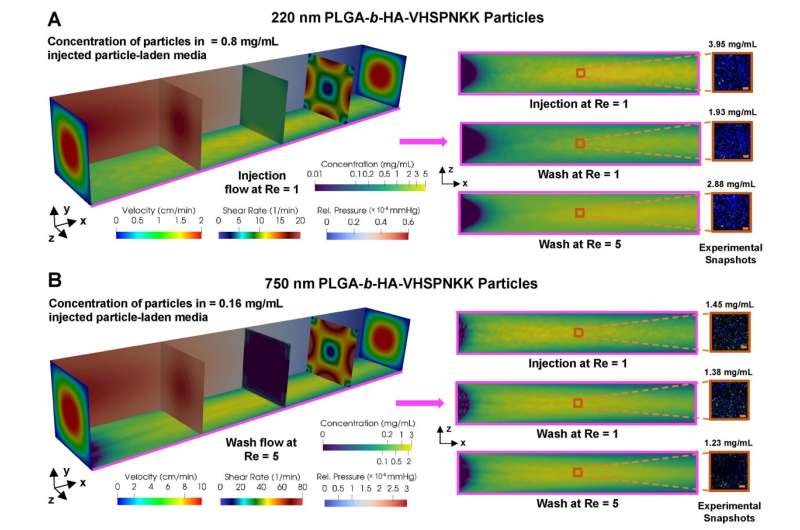
Chamber flow simulation for particle adhesion for 220 nm particles (top) and for 750 nm particles (bottom). Larger particles show greater retention after the wash stage than smaller particles. Credit: The Grainger College of Engineering at the University of Illinois Urbana-Champaign
Despite gaining a bad rap in mainstream media in recent years, nanoparticles have been successfully used for decades in targeted drug delivery systems. Drug molecules can be encapsulated within biodegradable nanoparticles to be delivered to specific cells or diseased tissues. However, blood flow dynamics can significantly affect the nanoparticle’s ability to bind at the target site and stay adhered long enough for the drug to be released.
Drawing inspiration from civil, mechanical, electrical and chemical engineering, University of Illinois Urbana-Champaign professors Arif Masud and Hyunjoon Kong have developed and tested a new mathematical model to accurately simulate the effects of blood flow on the adhesion and retention of nanoparticle drug carriers. The model closely corresponded to in-vitro experiments, demonstrating the impact that model-based simulations can have on nanocarrier optimization. In turn, this will accelerate drug design and patient-specific treatment.
The results of this research were recently published in the Proceedings of the National Academy of Sciences.
While treatments involving therapeutic drugs delivered to diseased tissues through the bloodstream have been effective, it is still unclear how much blood flow dynamics can affect the retention of nanoparticle drug carriers at target sites, which may be vastly different between animal models and humans. There are numerous factors that can affect an individual’s blood flow rate including their age, sex and level of physical activity, making it a very complex problem.
“Take a high-rise structure: there are many pipes and many angles, but water reaches every point of the building,” Masud explains. “Likewise, we have a similar network in our body but the ‘pipes’ are moving and bending all the time. The major contribution of this work is the development of a technique that can be used for optimizing drug delivery by figuring out flow rate, transportation to a specific point and attachment of the nanocarrier to that site.”
Kong adds, “There have been studies using mouse models and in-vitro tissue models. However, we have been designing nanoparticles mostly by trial and error. This is the first kind of demonstration where there is a more systematic, robust design of nanoparticles, under the guidance of physics.”
Masud and his team had been working on a mathematical model for blood flow for some time, but the model and experimental data did not produce the same results because they were assuming that the flow takes place in an idealized environment. They realized that they needed to bring in new ideas to get matching results.
First, the endothelial cell surface—the single cell layer that lines blood vessels—is not smooth like polished glass at the microscale. To adjust for this roughness, they incorporated an asperity model from mechanical engineering, which accounts for deformation when materials in contact are subject to force. Such a model is typically used for metals, but the researchers modified it for cellular materials.
Then, to attract nanocarriers from the bulk blood flow to the endothelial surface to then penetrate the diseased tissue, they used the concept of Lorentz forces from electrical engineering. Rather than a magnetic attraction, they exploited protein-protein attraction by coating the nanocarrier with the same protein excreted by the diseased tissue at the target site.
Finally, Masud’s team actually drew inspiration from an old civil engineering paper that investigated surface formation and deposition of sand particles on the Thames riverbed. They used this to create a model for particle flow in the boundary layer region.
“We derived these new ideas from very different diverse fields of engineering and the model started working,” Masud says.
Masud’s team first developed the mathematical model and then to refine it, Kong’s group ran experiments in carefully designed bio-chambers layered with endothelial cells. Nanoparticles were injected at a rate that replicated the arterial system and then flushed during a wash cycle to determine the concentration of remaining particles. Based on the results, the model was further optimized until simulations and experiments yielded similar results.
“The model is very general and can be applied to any kind of disease, different shapes of nanoparticles and different drugs,” Masud explains. “The beauty of the computer model is that we can optimize drug design and treatment in a digital environment and apply it to a specific patient.”
Using advanced imaging technology such as MRI and CT, the arterial structure of a patient can be recreated while also including their specific blood pressure, blood composition and viscosity. “We can create a digital twin of a living human to optimize the drug for that patient,” Masud says.
This can substantially shorten the time to find an optimized treatment protocol for a given patient, which can take months, even a year or more. With this model, simulations can be performed on supercomputers in as little as 24 to 48 hours.
Further, Masud and Kong were also able to simulate the effect of nanoparticle size and found that larger particles actually performed better at adhesion and retention at the endothelial layer. Researchers have generally focused on smaller particles so that they could go through smaller capillaries and get to the target site. “But one of the interesting findings from the simulation and experimentation was a significant loss of particles due to external flow for small diameter nanoparticles,” Kong says.
The simulation showed that 200 nanometer particles had detachment issues and would be washed away with external flow. Increasing the diameter to 1000 nanometers made the nanoparticles too big for transport. But 700 nanometers was the “Goldilocks” size and optimized attachment of particles at the vascular wall.
This interesting finding highlights the importance of simulation in drug design and delivery. Kong says, “Using a mouse model doesn’t always seem to work well for humans. We have very different physiological properties in terms of blood flow. Overall, simulation can be a very powerful tool.”
More information:
Shoaib A. Goraya et al, Modeling of spatiotemporal dynamics of ligand-coated particle flow in targeted drug delivery processes, Proceedings of the National Academy of Sciences (2024). DOI: 10.1073/pnas.2314533121
Citation:
Simulating blood flow dynamics for improved nanoparticle drug delivery (2024, June 27)
retrieved 28 June 2024
from https://phys.org/news/2024-06-simulating-blood-dynamics-nanoparticle-drug.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.
>>> Read full article>>>
Copyright for syndicated content belongs to the linked Source : Phys.org – https://phys.org/news/2024-06-simulating-blood-dynamics-nanoparticle-drug.html