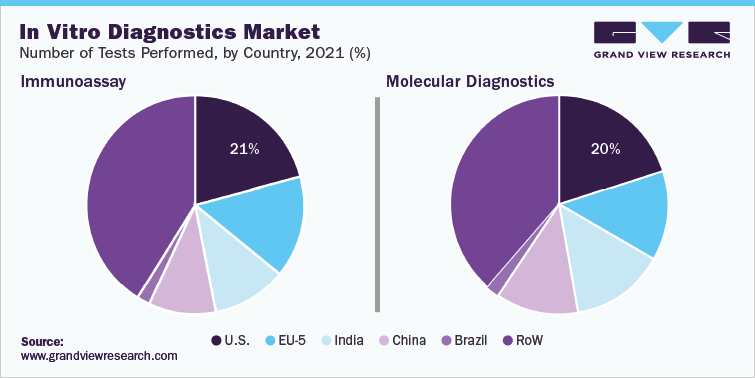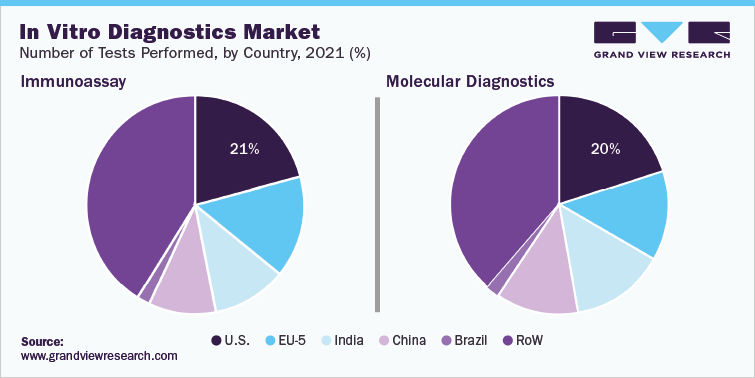
In vitro diagnostics and IVD quality control industry data book covers in vitro diagnostics (IVD) and IVD quality control market.
Global In Vitro Diagnostics and IVD quality control industry data book is a collection of market sizing & forecasts insights, regulatory & technology framework, pricing intelligence, volumetric analyses, competitive benchmarking analyses, macro-environmental analyses studies. Within the purview of the database, such information is systematically analyzed and provided in the form of summary presentations and detailed outlook reports on individual areas of research. The following data points will be included in the final product offering in two reports and one sector report overview.
In Vitro Diagnostics (IVD) Market Analysis & Forecast
The global in vitro diagnostic market size was valued at USD 111.67 Billion in 2021 and is projected to grow at a compound annual growth rate (CAGR) of 0.2 % from 2022 to 2030. IVD renders accurate & effective results and has indispensable applications in the field of disease diagnosis. Eventually, quality control services have become requisite in accredited medical or clinical laboratories. In vitro diagnostics shows significant potential for growth driven by an aging global population that requires accurate diagnosis, increased demand for targeted cancer therapies supported by Companion Diagnostic (CDx) devices, and rising demand for infectious diseases diagnostics due to frequent viral/bacterial outbreaks such as COVID-19.
In addition, NGS-based genetic prenatal tests are becoming more common, and adoption of these tests is rising due to their low false-positive rates. Hence, FDA recently issued guidance to improve oversight of test quality, accuracy, safety, and medical benefit. Key players such as Illumina, Inc. have NGS-based prenatal screening tests that allow detection of common abnormalities like Down syndrome. Furthermore, the number of people suffering from congenital heart disease is increasing, requiring medical professionals to develop IVD tools for accurate diagnoses. According to CDC, congenital heart defects are the most common birth defects in the U.S., affecting approximately 1% of births every year.
The use and demand of Point-of-Care (PoC) tests and devices are rising owing to increased demand for rapid identification of diseases in close proximity to patients to facilitate faster decision-making. This trend is pushing manufacturers to launch small, transportable, fast, and easy-to-use instruments, making use of these instruments easier in non-laboratory settings. For instance, in July 2021, QuantuMDx announced the launch Q-POC system, which offers rapid PoC molecular diagnostics advantageous for settings, such as ICUs, clinics, and birthing centers.
IVD Quality Control Market Analysis & Forecast
The global in vitro diagnostics quality control market size was estimated at USD 1.12 Billion in 2021 and is expected to grow at a compound annual growth rate (CAGR) of 2.0% from 2022 to 2030. In IVD quality control, immunochemistry led the global market in 2021 and accounted for a revenue share of more than 35.00%. Key applications of the immunochemistry include detection of infectious microorganisms, such as viruses, bacteria, and fungi, by detecting the presence of their toxins and coat antigens.
Diagnostics laboratories are in demand owing to the increasing prevalence of diseases such as infectious diseases, cardiovascular conditions, and diabetes. To meet the industry demand, many private and public laboratories are undergoing accreditation procedures, which is creating a surge for IVD quality control measures. As of May 2022, the quality standards of 330,000 laboratories in the U.S. are regulated under the Clinical Laboratory Improvement Amendments (CLIA) by the CMS, making quality management crucial.
Order Free Sample Copy of “In Vitro Diagnostics And IVD Quality Control Industry Data Book – In Vitro Diagnostics & IVD Quality Control Market Size, Share, Trends Analysis, And Segment Forecasts, 2022 – 2030” published by Grand View Research
Key players operating in the In Vitro Diagnostics And IVD Quality Control Industry are –
• ABBOTT
• BIOMÉRIEUX SA
• BIO-RAD LABORATORIES, INC.
• BECTON, DICKINSON AND COMPANY
• SIEMENS HEALTHINEERS AG
• QIAGEN
• QUIDEL CORPORATION
• F. HOFFMANN-LA ROCHE LTD.
• SYSMEX CORPORATION
• CHARLES RIVER LABORATORIES
• QUEST DIAGNOSTICS
• AGILENT TECHNOLOGIES, INC.
• DANAHER CORPORATION
• Bio-Rad Laboratories, Inc.
• Thermo Fisher Scientific, Inc.
• Sero AS.
• Sun Diagnostics, LLC
• Ortho Clinical Diagnostics, Inc.
• Randox Laboratories Ltd
• Helena Laboratories Corporation
• SeraCare Life Sciences, Inc.
• Technopath Clinical Diagnostics
Grand View Research, Inc. is a market research and consulting company that provides off-the-shelf, customized research reports and consulting services. To help clients make informed business decisions, we offer market intelligence studies ensuring relevant and fact-based research across a range of industries, from technology to chemicals, materials and energy. With a deep-seated understanding of varied business environments, Grand View Research provides strategic objective insights. For more information, visit http://www.grandviewresearch.com
>>> Read full article>>>
Copyright for syndicated content belongs to the linked Source : PRSync – http://prsync.com/grand-view-research-inc/in-vitro-diagnostics-and-ivd-quality-control-industry-quality-controls-crucial-role-in-ivd-3948115/