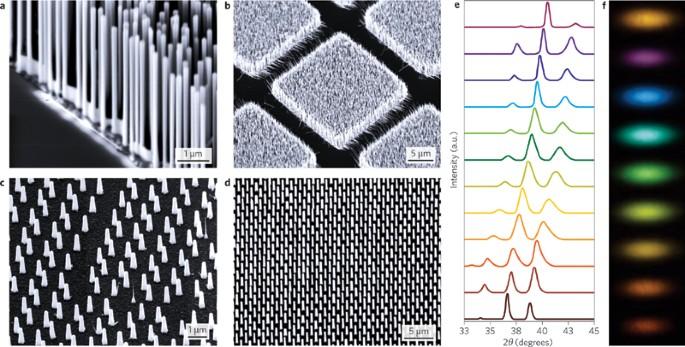
Scientists have taken a major step toward restoring vision by successfully using nanowires to replace lost retinal cells, a breakthrough that could revolutionize treatments for degenerative eye diseases. The new approach, detailed in a recent study published by the American Association for the Advancement of Science (AAAS), demonstrates how nanoscale structures can integrate with neural tissue to restore the complex functions of the retina. This cutting-edge technology offers hope for millions suffering from vision loss, marking a significant advance in regenerative medicine and bioengineering.
Nanowires Demonstrate Promise in Restoring Vision by Replacing Damaged Retinal Cells
Recent breakthroughs in nanotechnology have brought to light the potential of nanowires as a viable solution for retinal degeneration-a leading cause of vision loss worldwide. These ultra-fine, conductive structures effectively mimic the function of lost retinal cells by interfacing seamlessly with existing neurons, transmitting visual signals to the brain. Unlike traditional retinal implants, nanowires offer a more integrated and less invasive approach, reducing inflammation and promoting long-term stability within the delicate retinal tissue.
- High biocompatibility: Nanowires encourage cell growth and minimize immune response.
- Enhanced signal transmission: Their nano-scale size allows precise electrical interfacing with neuronal circuits.
- Longevity: Early trials suggest robust durability in ocular environments.
| Property | Nanowires | Conventional Implants |
|---|---|---|
| Size | 100 nm | Micrometer scale |
| Integration | Neuron-level | Tissue-level |
| Inflammation Risk | Low | High |
| Functional Lifespan | Years (projected) | Months to years |
Researchers Uncover Mechanisms Behind Nanowire Integration in Retinal Tissue
The research team has identified critical processes enabling nanowires to integrate seamlessly into damaged retinal tissue, a breakthrough that could revolutionize treatments for vision loss. These tiny conductive structures mimic the intricate connectivity of lost retinal cells, effectively restoring signal transmission to the brain. Through advanced imaging and electrophysiological techniques, scientists observed that the nanowires form stable synapse-like junctions within the delicate neural network, allowing for efficient communication and functional recovery.
Key mechanisms discovered include:
- Selective adherence of nanowires to surviving retinal neurons
- Promotion of synaptic plasticity facilitating signal acceptance
- Minimization of inflammatory response ensuring biocompatibility
| Mechanism | Impact on Integration |
|---|---|
| Adherence | Ensures precise positioning of nanowires |
| Synaptic Plasticity | Facilitates neural signal transmission |
| Inflammation Control | Prevents tissue rejection and scarring |
Experts Call for Accelerated Clinical Trials to Assess Safety and Efficacy of Nanowire-Based Treatments
Leading scientists emphasize the urgent necessity to expedite clinical trials focused on nanowire-based therapies designed to restore damaged retinal cells. Early laboratory studies reveal that these nanoscale structures can successfully mimic the complex architecture and function of lost photoreceptors, promising a revolutionary shift in treating degenerative eye conditions. However, experts stress that comprehensive testing in human subjects is critical to validate both safety and long-term efficacy before widespread adoption.
Key challenges ahead include understanding potential immune responses, optimizing integration with existing neural circuits, and ensuring reliability across diverse patient populations. To guide future research priorities, specialists highlight several focal areas:
- Biocompatibility assessments to prevent adverse tissue reactions
- Functional outcome evaluations measuring vision restoration metrics
- Scaling manufacturing processes for clinical-grade nanowires
| Trial Phase | Primary Objective | Estimated Duration |
|---|---|---|
| Phase I | Safety and Tolerability | 6-12 months |
| Phase II | Preliminary Efficacy | 12-18 months |
| Phase III | Comparative Effectiveness | 18-24 months |
In Retrospect
As research into nanowire technology advances, the prospect of restoring vision for millions affected by retinal diseases moves closer to reality. While challenges remain before clinical application, these groundbreaking findings mark a significant step forward in regenerative medicine. Continued exploration and testing will determine how soon nanowire-based therapies might transform the landscape of ocular treatment, offering new hope to patients worldwide.