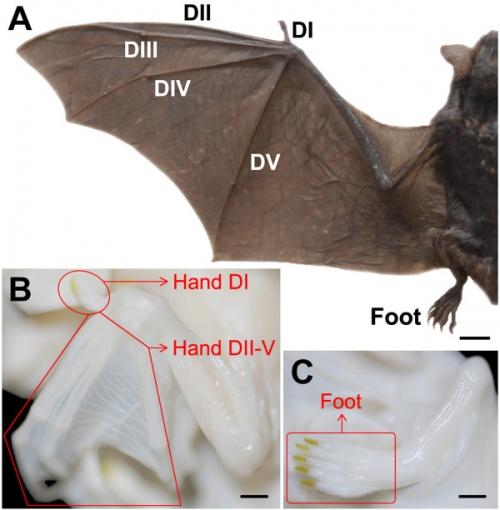
A groundbreaking study published in Nature sheds new light on the evolutionary innovations behind bat wing development. By employing comparative single-cell analyses, researchers have uncovered how a conserved genetic program has been repurposed over millions of years to shape the unique anatomy of bat wings. This discovery not only deepens our understanding of bat evolution but also highlights the dynamic flexibility of genetic pathways in driving the diversity of life’s forms.
Comparative Single-Cell Analysis Sheds Light on Bat Wing Evolution
The comprehensive single-cell analyses conducted across multiple species have uncovered a fascinating evolutionary twist in the genetic blueprint responsible for limb formation. By charting the activity of individual cells during key developmental stages, researchers identified that bats have repurposed a highly conserved gene program, originally shared with other mammals, to facilitate the growth of their uniquely elongated wings. This evolutionary innovation highlights how small shifts in gene regulation, rather than wholesale changes in genetic code, can produce dramatic morphological adaptations.
Key findings emphasize the role of cell-type-specific gene expression changes and the dynamic interplay of signaling pathways that drive bat wing expansion. Some notable aspects include:
- Enhanced proliferation of connective tissue progenitors in bat forelimbs
- Modulation of growth factor responses absent in other mammalian limbs
- Retention of a core genetic architecture that supports diverse limb morphologies
| Species | Key Gene Expression Feature | Functional Outcome |
|---|---|---|
| Bats | Upregulated connective tissue progenitors | Wing elongation |
| Mouse | Standard limb growth factor profile | Normal forelimb size |
| Chicken | Distinct mesenchymal signaling signature | Wing development without elongation |
Conserved Gene Programmes Show New Roles in Limb Development
Recent investigations utilizing comparative single-cell transcriptomics have uncovered that gene networks long considered conserved across vertebrates are being dynamically retooled during bat wing morphogenesis. These gene programmes, traditionally associated with fundamental developmental processes, demonstrate surprising plasticity, revealing novel functions that contribute to the extraordinary diversification of limb structures. Notably, key regulatory modules that guide early limb patterning in mammals exhibit unique temporal and spatial expression shifts in bats, underpinning the elongation and digit specialization crucial for powered flight.
The study highlights several pivotal genes and pathways – including members of the Hox cluster, Bmp, and Fgf families – repurposed to orchestrate differential growth rates and tissue morphogenesis in the bat wing. A summarized comparative expression pattern in forelimb versus hindlimb cells of bats and mice reveals the adaptive rewiring at the single-cell level:
| Gene | Forelimb Expression (Bat) | Hindlimb Expression (Bat) | Forelimb Expression (Mouse) |
|---|---|---|---|
| Hoxd13 | High | Low | Moderate |
| Fgf8 | Elevated | Moderate | Consistent |
| Bmp2 | Moderate | High | Moderate |
- Temporal shifts in gene activation correlate with elongated digit growth phases unique to bats.
- Tissue-specific modulation of signaling pathways facilitates membrane formation between digits.
- Evolutionary repurposing of canonical limb genes supports morphological innovations necessary for powered flight.
Experts Call for Expanded Single-Cell Studies to Unlock Evolutionary Secrets
Recent findings presented in Nature underscore the complexity and dynamism of genetic programs conserved across species yet adapted for specialized functions. By leveraging comparative single-cell analyses, researchers have illuminated how an ancient gene regulatory network has been evolutionarily retooled to guide the unique morphogenesis of bat wings. This discovery not only deepens our understanding of developmental biology but also accentuates the potential of single-cell sequencing technologies to unravel the nuances of evolutionary innovation at an unprecedented resolution.
Experts emphasize the critical need for expanding single-cell studies across diverse organisms to fully decode the genetic and cellular mechanisms driving phenotypic diversity. Key areas highlighted include:
- Deciphering cellular heterogeneity that defines organ-specific adaptations.
- Mapping conserved versus novel gene expression patterns to trace evolutionary trajectories.
- Integrating multi-omics data to build comprehensive cellular atlases linking genotype to phenotype.
| Feature | Bat Wing Cells | Mouse Limb Cells |
|---|---|---|
| Gene Program Activity | Rewired for elongation | Standard limb patterning |
| Cellular Diversity | Expanded mesenchymal subtypes | Conserved mesenchymal types |
| Developmental Timing | Prolonged gene expression phase | Typical expression window |
Wrapping Up
The groundbreaking study published in Nature sheds new light on the intricate genetic choreography behind bat wing development, revealing how a deeply conserved gene program has been evolutionarily repurposed to enable flight. By leveraging cutting-edge single-cell analyses, researchers have not only unraveled the cellular diversity shaping these unique limbs but also opened doors to understanding evolutionary innovation at a molecular level. As science continues to delve into the genomic blueprints of diverse species, findings like these underscore the dynamic ways nature retools existing genetic frameworks to forge novel anatomical features – offering fresh perspectives on evolution’s creative potential.