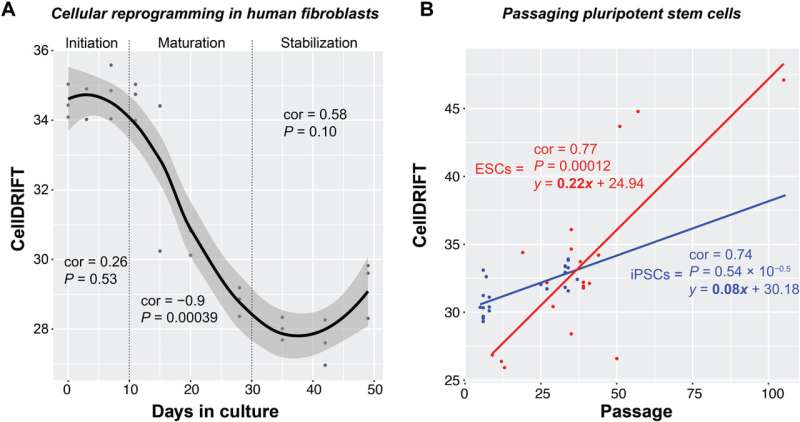
CellDRIFT is turned back upon reprogramming in human fibroblasts, but is not stabilized with additional passages. (A) Scatterplot showing reprogramming trajectory in CellDRIFT from OSKM reprogrammed human dermal fibroblasts from GSE54848, analyzed separately for initiation, maturation, and stabilization phases. (B) Scatterplot demonstrating that passaging increases CellDRIFT in pooled iPSCs reprogrammed from human dermal fibroblast’s via OSKM and ESCs. Note that 11 different iPSC lines were included, which were generated from 11 clones, and 15 ESC lines were included (GSE31848). Correlation and statistical significance were determined via Pearson correlations in each phase and in the extended passaging plot. Credit: Science Advances (2023). DOI: 10.1126/sciadv.adf4163
For nearly every disease, age is a major risk factor, and cancer is no exception. Between the ages of 25 and 65, an individual’s risk of developing cancer skyrockets by 4,000%. A new study explores a “fingerprint” in cells associated with cancer and aging to better understand if researchers can predict who is at risk before cancer develops.
Scientists knew there was a link between aging and cancer but had little understanding of the key features of aging that may be informative about disease risk. DNA methylation (DNAm) is a biological process through which the DNA molecule undergoes alterations of the chemicals attached to it.
Previous research has observed cellular DNAm changes that are common to both aging and cancer risk. Now, a Yale-led team has developed cell models which isolate a DNAm signal or fingerprint related to aging and cancer, called CellDRIFT, to better understand how it drives disease.
Through analysis of clinical tissue samples, they found that CellDRIFT is escalated in aging tissues, cancerous tissues, and even normal tissues sampled from patients with cancer. The findings provide a clearer picture of the unseen progressive steps occurring inside cells that eventually lead to cancer, and could pave the way for earlier detection of the disease. The team published its findings in Science Advances on July 19.
“We found that we could quantify age as more than just a number,” says Christopher Minteer, Ph.D., who was first author of the study in the lab of Morgan Levine, Ph.D., now adjunct assistant professor of pathology at Yale and a principal investigator at the Altos Labs San Diego Institute of Science.
Is cancer driven by more than bad luck?
A 2015 study published in Science looked at the relationship between cancer risk and cancer tissue type. In particular, the authors were looking to see if higher incidences of cancer could be explained by stem cell division rates.
“They were interested in the idea that the longer we live, the more years we have to accumulate potential ‘bad luck events’ that could be drivers toward an outcome over time,” says Minteer. These bad luck events—the random mutations that drive cancer risk—are challenging to predict or prevent. This ideology became known in the pathology world as the “bad luck hypothesis.”
However, the Yale team that did the new study believed that an individual’s risk of cancer involves factors beyond simple bad luck. They became interested in looking at measurable environmental influences that could contribute to cancer and help researchers make better-informed predictions about cancer risk.
Scientists model cellular ‘fingerprint’ associated with aging and cancer
In a 2022 study, the lab explored changes in DNAm associated with aging. Now, in the current study, the team wanted to build upon its prior work and figure out how to model these DNAm changes. First, they cultured human cells derived from the brain, called astrocytes. For some of these cells, they induced a gene called human telomerase reverse transcriptase (hTERT), which causes the cells to become “immortal”—in other words, they had been manipulated to divide indefinitely, similar to tumorous cells.
Over three months, they took a subset of cells from their cultures and extracted DNA from them. Using a de novo computational training platform, they then quantified DNAm signals and identified a particular fingerprint, CellDRIFT, that significantly increased with aging. “We were able to show that we can isolate a signal that is associated with aging and also is known to be important in cancer,” says Minteer.
CellDRIFT linked to poorer outcomes in patients with cancer
Next, the team used clinical tissues to study the relationship between CellDRIFT and cancer outcomes. Through analyzing tissues of the thyroid, breast, lung, pancreas, and colon, they found that cancerous tissues showed an increased CellDRIFT compared to healthy control tissues. Then, utilizing data from a breast cancer cohort, they also discovered a significant relationship between the signal and survival, correlating CellDRIFT with poorer health outcomes and potentially helping researchers predict how aggressive a particular cancer is.
Furthermore, the researchers biopsied healthy tissue in breast cancer patients approximately three centimeters away from the tumor. Interestingly, even in the healthy tissue, they found increased CellDRIFT compared with control tissue from healthy patients. “This gives us some hope that we could perhaps assay some level of risk prior to disease,” says Minteer.
Finally, the team collected 29 tissue types from four post-mortem patient donors to better understand variations in CellDRIFT across the samples. They found significant correlations among CellDRIFT, cancer incidence, and stem cell-division rates. “We were able to add context to the 2015 study that proposed that different tissue types have different cancer risks,” says Minteer. “We were able to show that there’s more to cancer risk that random variation from stem cell mutations.”
The researchers hope the study will help scientists better understand how to delay the onset of chronic diseases such as cancer that appear related to aging and help people live longer, healthier lives.
More information:
Christopher J. Minteer et al, More than bad luck: Cancer and aging are linked to replication-driven changes to the epigenome, Science Advances (2023). DOI: 10.1126/sciadv.adf4163
Citation:
Cellular ‘fingerprint’ may offer early warning of cancer risk (2023, July 24)
retrieved 24 July 2023
from https://medicalxpress.com/news/2023-07-cellular-fingerprint-early-cancer.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.
>>> Read full article>>>
Copyright for syndicated content belongs to the linked Source : Medical Xpress – https://medicalxpress.com/news/2023-07-cellular-fingerprint-early-cancer.html
