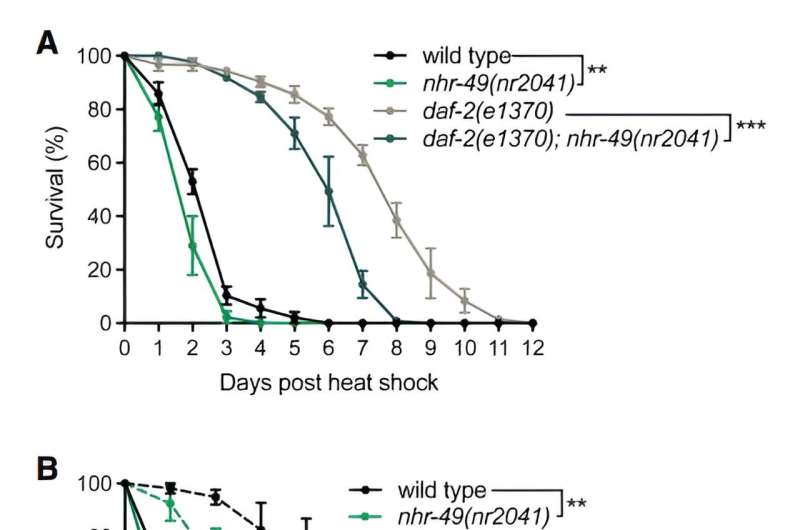
NHR-49 is important in multiple paradigms of stress resilience. (A) Heat stress survival of wild type and daf-2(e1370) mutants crossed into nhr-49(nr2041). (B) Heat stress survival of wild type and nhr-49(nr2041) mutants grown on OP50 bacteria (fed) or deprived of food source for 16 h prior to the heat stress (starved). (C) Heat stress survival of wild type and glp-1(e2141) temperature-sensitive mutants crossed into nhr-49(nr2041). Credit: Genes & Development (2024). DOI: 10.1101/gad.351829.124
Northwestern investigators have identified a novel transcription factor that regulates a signaling mechanism utilized by a fertilized embryo to protect its mother from cellular and environmental stress, according to a study to published in the journal Genes & Development.
The findings have implications for promoting healthy aging by therapeutically targeting age-related protein dysregulation and misfolding, which can lead to the development of many neurodegenerative disorders.
“Our work is really trying to understand in aging what are the earliest events that we can detect to restore proteostasis, because proteostasis controls the health of every protein. The proteostasis network that controls protein synthesis, folding and clearance is the only cellular pathway that we know of that can prevent or enhance the clearance of amyloid formation,” said Richard Morimoto, Ph.D.
Morimoto is a professor in the Ken and Ruth Davee Department of Neurology and the Bill and Gayle Cook Professor of Biology in the Weinberg Colleges of Arts and Sciences, and was senior author of the study.
The health of an organism relies on a functional proteome, or the entire set of proteins expressed in every cell of an organism. As an organism naturally ages, proteins exhibit a higher risk of misfolding and aggregating to form amyloid, which plays a key role in the development of many neurodegenerative diseases in humans, including Alzheimer’s disease, Parkinson’s disease and cystic fibrosis.
This “proteostasis collapse” is a phenomenon previously discovered by Morimoto and colleagues by studying C. elegans models of neurodegenerative diseases. C. elegans is a transparent roundworm whose biochemical makeup is similar to that of humans and is used by scientists to better understand human biology, including aging and disease development.
In a recent study, Morimoto’s group found using C. elegans models of neurodegenerative diseases that a damaged fertilized embryo protects the mother from cellular stress by sending her molecular signals to promote her overall health so that she can produce future generations of healthy eggs.
The results of a genetic screen performed in C. elegans revealed that when the vitelline layer—the outer shell of the egg—is damaged, the embryo signals to the mother to prevent proteostasis collapse and to suppress protein aggregation.
“It was stunning. It was, in a sense, a novel form of transgenerational signaling,” Morimoto said. “That was intriguing because this is about communicating from one tissue, the egg, to the mother.”
In the current study, using advanced transcriptomics techniques, Morimoto’s team found that this signaling pathway is regulated by a transcription factor called NHR-49, which is known to support gene expression in all metazoans that use eggs, including humans, and regulates lipid metabolism.
Lipid metabolism is the breakdown and storage of fatty acids for the cell to use as energy and also supports the conversion of lipids for constructing the cell membrane.
The findings establish NHR-49 as a key regulator that connects lipid homeostasis and cellular resilience to proteotoxic stress. This signaling pathway could therefore serve as a biomarker and therapeutic target for regulating proteostasis and, ultimately, promoting healthy aging, according to Morimoto.
“The major emphasis for the future is to now convert this into predictable models that for which we could then target that pathway or that set of genes,” Morimoto said.
The current study is part of a larger effort led by Morimoto to improve the understanding of how proteostasis regulates healthy aging and to prevent diseases of protein misfolding.
“Our ability to identify the machinery and understand what is optimal allows us to then understand how to rejuvenate the cell, the tissue, and the organism against the threats of aging,” Morimoto said.
More information:
Ambre J. Sala et al, Nuclear receptor signaling via NHR-49/MDT-15 regulates stress resilience and proteostasis in response to reproductive and metabolic cues, Genes & Development (2024). DOI: 10.1101/gad.351829.124
Citation:
Embryo’s signaling mechanism may promote healthy aging, combat neurodegenerative diseases (2024, July 1)
retrieved 1 July 2024
from https://medicalxpress.com/news/2024-07-embryo-mechanism-healthy-aging-combat.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.
>>> Read full article>>>
Copyright for syndicated content belongs to the linked Source : Medical Xpress – https://medicalxpress.com/news/2024-07-embryo-mechanism-healthy-aging-combat.html
