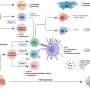
Immune and bone marrow environment changes during the MDS course. Credit: HemaSphere (2024). DOI: 10.1002/hem3.64
Researchers from King’s College have led research that has resulted in the publication of first-of-their-kind guidelines that seek to standardize how clinicians measure the immune response of patients who have myelodysplastic syndromes (MDS), a type of hard to treat blood cancer.
The new guidelines, published in HemaSphere, will help hematologists identify which patients could potentially respond to specific types of therapies, and also better categorize patients based on their type of immune response (autoimmune versus autoinflammatory), which affects the choice of therapy and clinical outcome.
The recommendations have been endorsed by the European Hematology Association (EHA), which has also awarded further funding (Innovation Grant 2024) over a two-year period to test their feasibility and accuracy. This grant was awarded during the opening ceremony of EHA 2024 congress in Madrid, Spain.
“I am delighted that we have published the first consensus-based guideline for immune monitoring in MDS as part of our i4MDS consortium activities. I am also very grateful to the EHA for awarding us an Innovation Grant to start the clinical assessment and implementation of this approach.
“This is an important step toward a more standardized way of immune monitoring in MDS and subsequently more ‘immune-informed’ clinical trials in MDS patients,” says Dr. Shahram Kordasti, reader and group leader in applied cancer immunopathology.
The guidelines were created by the International Integrative Immunology for MDS (i4MDS)—an international consortium created and led by Dr. Shahram Kordasti, Reader and Group Leader in Applied Cancer Immunopathology. The consortium aims to utilize international expertise to foster a deeper understanding of the immune mechanisms underlying MDS. This, in turn, will aid efforts in drug development toward its treatment.
MDS are a group of cancers in which immature blood cells in the bone marrow do not mature or become healthy blood cells. Instead of developing normally, the blood cells die in the bone marrow or just after entering the bloodstream.
People over the age of 60 are at increased risk of MDS, and the prevalence of these diseases is growing due to a rapidly aging population worldwide. There are various risks associated with MDS, including increased likelihood of infections, anemia and bleeding and bruising. MDS can also progress to Acute Myeloid Leukemia (AML), which is a more aggressive and difficult-to-treat form of leukemia.
Immunotherapy is a powerful tool that has been effective against several cancers, such as lung and skin cancers. It harnesses the body’s immune system and directs it to fight cancer cells.
The role of the immune system in the pathophysiology of MDS is firmly established. However, routine immune monitoring for these patients is still not a common practice. Furthermore, a universally accepted protocol for which components of the immune system should be monitored had not been agreed upon.
The new guidelines give recommendations around flow cytometry, a powerful analytical tool used to measure and analyze the physical and chemical characteristics of individual cells as they flow through a beam of light. In flow cytometry, “panels” refer to groups of specific antibodies used to detect and measure various cellular markers that can characterize different cell populations. The new guidelines give recommendations for seven panels for measuring immune response in MDS.
More information:
Cristina A. Tentori et al, Immune‐monitoring of myelodysplastic neoplasms: Recommendations from the i4MDS consortium, HemaSphere (2024). DOI: 10.1002/hem3.64
Citation:
First guideline on immune monitoring in patients with a type of blood cancer (2024, July 12)
retrieved 14 July 2024
from https://medicalxpress.com/news/2024-07-guideline-immune-patients-blood-cancer.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.
>>> Read full article>>>
Copyright for syndicated content belongs to the linked Source : Medical Xpress – https://medicalxpress.com/news/2024-07-guideline-immune-patients-blood-cancer.html