
Striking the right balance: in nerve cells, the hormone insulin and the protein PINK1 control whether cellular power plants are kept running or whether they are shut down. Credit: MPI f. Biological Intelligence/ Julia Kuhl
The hormone insulin controls many cellular processes and adapts them to the body’s current energy supply. One of the insulin-regulated processes is the quality control of mitochondria in neurons, Angelika Harbauer and her team at the Max Planck Institute for Biological Intelligence have discovered.
When sufficient energy is available in the body, insulin facilitates the elimination of defective mitochondria. When energy is scarce or when the insulin signal is interrupted, mitochondrial recycling is reduced and cells continue to use their old power plants, even potentially damaged ones. The continued operation of faulty mitochondria could affect aging processes and neurological diseases.
The team’s research appears in Nature Metabolism.
Nerve cells place special demands on their energy supply. Due to their extensive branching and their high energy needs, they keep a close watch on their cellular power plants, the mitochondria. The cells have to ensure that there are always sufficient mitochondria available in their long extensions, the axons, where the power plants fuel the cell’s communication with its neighboring cells. This is why neurons transport mitochondria even to the cells’ most remote locations.
Harbauer’s earlier research had shown that mitochondria carry along the blueprints of the PINK1 protein on their journey through the neuron.
“PINK1 is a key protein that acts when mitochondria need to be removed because they are no longer functioning correctly,” explains the Max Planck research group leader. “It can mark mitochondria for recycling and is precisely controlled by the cells.” A failure to keep PINK1 in check could lead to a shortage of mitochondria, whereas the continued operation of defective cellular power plants can damage a cell.
A hormone with many roles
Harbauer and her team have now uncovered that the hormone insulin is involved in mitochondrial quality control in neurons. Insulin is well-known for its role in regulating a cell’s sugar uptake. It also controls many processes inside cells to precisely adjust them to the body’s current energy supply.
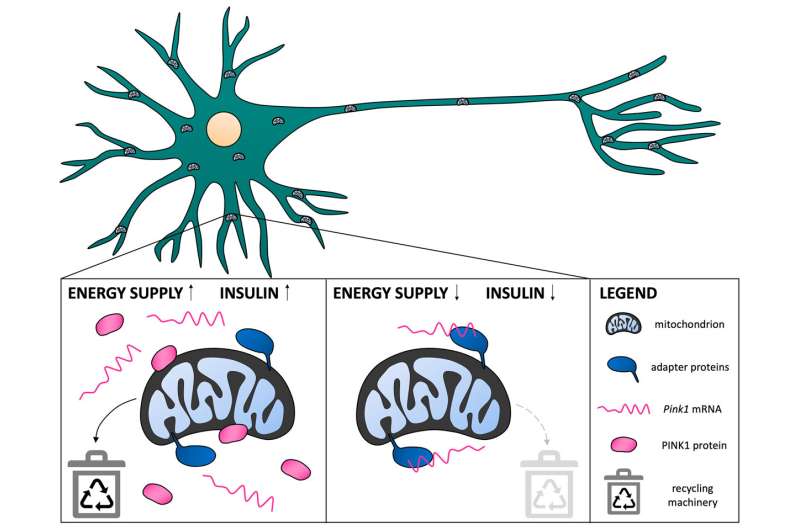
A signal from the insulin receptor (left panel) leads to the release of Pink1 mRNA molecules from the mitochondrial surface, where the mRNAs are bound for transport with the help of adapter proteins. The cell can produce PINK1 protein and defective mitochondria are efficiently removed. If the insulin signal is missing (right panel), the mRNA molecules remain firmly bound to the mitochondria and are unavailable for the production of PINK1 protein. Due to the decreased production of PINK1 protein, the recycling of mitochondria is reduced. (Illustration not to scale.). Credit: Max Planck Institute for Biological Intelligence / Tabitha Hees
In the case of mitochondrial recycling, this works as follows: If sufficient energy is available, a signal is transmitted from the insulin receptor on the cell surface to the mitochondria. Here, PINK1 blueprints are stored as mRNA molecules. When the insulin signal arrives, the blueprints are released by the mitochondria and the cell can produce additional PINK1 protein. This ensures that defective mitochondria are efficiently eliminated. In case of an energy shortage, or if the insulin receptor signal is missing, the blueprints for PINK1 remain tightly bound to the mitochondria.
On the one hand, the tight binding to mitochondria allows the PINK1 blueprints to hitchhike far into the nerve cells’ long extensions. On the other hand, it reduces the availability of mRNA molecules for PINK1 production. PINK1 protein levels remain low and mitochondrial recycling is reduced—even though this can lead to the continued operation of damaged power plants.
“We had expected that the binding of the mRNA to the mitochondria would promote PINK1 production,” says Tabitha Hees, lead author of the study. “Surprisingly, our experiments showed that this is not the case. When energy levels are low, it is apparently more favorable for the cells to produce less PINK1 protein and to continue using potentially damaged mitochondria.”
Interrupted signaling with implications for health and aging
A similar situation can occur when the transmission of signals from the insulin receptor to mitochondria is disturbed due to disease. Defective insulin signaling is a hallmark of diabetes and has also been observed in the brain in connection with Alzheimer’s disease.
It is also known that inefficient mitochondrial quality control can contribute to various neurodegenerative diseases. “Our observations add to our understanding of how cellular energy supply, aging and neurodegenerative diseases are interrelated,” says Harbauer.
Next, the researchers aim to investigate what happens to the PINK1 blueprints once they are released from the mitochondria into the cell.
“We are particularly interested in finding out where the PINK1 protein is made, if not at mitochondria, and how it afterwards finds its way back to mitochondria,” says researcher Tabitha Hees. Only when these two steps are taken care of, PINK1 will initiate the recycling of faulty power plants to prevent them from damaging the nerve cell.
More information:
Insulin signaling regulates Pink1 mRNA localization via modulation of AMPK activity to support PINK1 function in neurons, Nature Metabolism (2024). DOI: 10.1038/s42255-024-01007-w
Citation:
In nerve cells, insulin regulates whether mitochondria are shut down or kept running (2024, March 19)
retrieved 19 March 2024
from https://medicalxpress.com/news/2024-03-nerve-cells-insulin-mitochondria.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.
>>> Read full article>>>
Copyright for syndicated content belongs to the linked Source : Medical Xpress – https://medicalxpress.com/news/2024-03-nerve-cells-insulin-mitochondria.html










