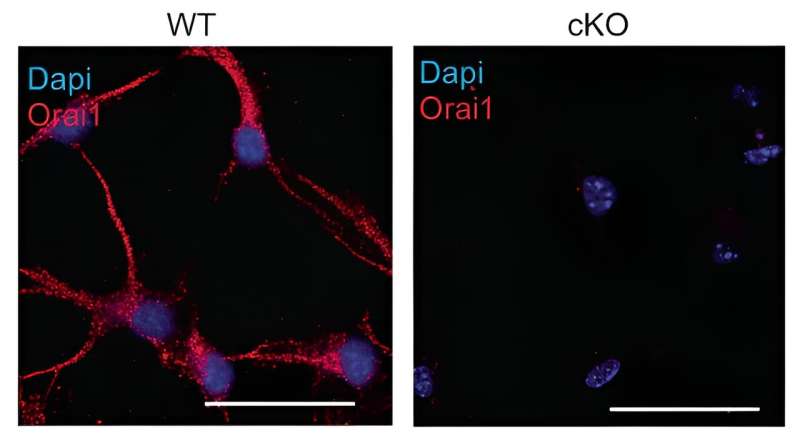
Immunohistochemistry of WT hippocampal astrocytes in culture showing expression of Orai1 in the soma and processes. Orai1 labeling (with a monoclonal antibody, Abcam, M266.1) is lost in astrocytes from Orai1 KO (Orai1fl/fl GFAP-Cre) mice. Scale bar: 25 µm. Credit: Nature Communications (2023). DOI: 10.1038/s41467-023-40968-6
Northwestern Medicine investigators have identified how a calcium channel in the nervous system contributes to brain inflammation, according to a study published in Nature Communications.
Astrocytes, the major glial subtype of the nervous system, mediate several important tasks in the brain, including clearing excess neurotransmitters at synapses, providing metabolic nutrients to neurons, and controlling the blood-brain barrier.
In addition to these well-established functions, it was recently recognized that astrocytes also cause neuroinflammation, which can cause significant tissue damage and lead to changes in animal behavior. Despite the abundance of astrocytes and widespread nature of neuroinflammation, little is known about the molecular checkpoints that control astrocyte-mediated brain inflammation, said Murali Prakriya, Ph.D., the Magerstadt Professor of Pharmacology and of Medicine in the Division of Allergy and Immunology, and senior author of the study.
Prakriya’s laboratory determined that a calcium channel, ORAI1, plays a key role in controlling astrocyte reactivity and their ability to produce and release inflammatory mediators. Because astrocyte activity is regulated by intracellular calcium, investigators first bred mice without the ORAI1 gene, which has been shown to control calcium signaling in many mammalian cells including immune cells and microglia.
Mice without ORAI1 in astrocytes were unable to effectively produce and release inflammatory cytokines, according to the study. The study also found that in the absence of ORAI1 signaling, cellular metabolism related to glycolysis and mitochondrial pathways were reduced.
Prakriya and his collaborators observed that mice lacking ORAI1 in astrocytes did not show elevated brain inflammation in response to an injection of inflammation-causing bacteria.
“In this way, ORAI1 regulates multiple interlinked cellular processes that drive inflammation in the brain,” Prakriya said.
Importantly, the ORAI1 astrocyte knockout mice were protected against inflammation-related behavioral depression, according to the study.
“It’s well established that people who suffer intense peripheral inflammation arising, for example, from infections or major surgery, show depression-like symptoms afterwards. So we asked, ‘What is the contribution of astrocyte activation and astrocyte calcium signaling for this behavioral depression?,'” said Prakriya, who is also a member of the Robert H. Lurie Comprehensive Cancer Center of Northwestern University. “This is novel for the field because the role of astrocyte calcium signaling in controlling brain inflammation has not really been explored.”
These findings confirm that ORAI1 plays an important role in regulating brain inflammation, Prakriya added. Because neuroinflammation is a common feature of many neurological diseases, the results may aid the quest for developing new types of therapeutics for mitigating neuroinflammation.
“When we looked at behaviors indicative of depression-like symptoms following the inflammatory challenge, we found that wild-type mice exhibited well-established depression-like behaviors of anhedonia and helplessness,” said Michaela Novakovic, Ph.D., a recent graduate of the Northwestern University Interdepartmental Neuroscience (NUIN) program and first author of the study.
“But when we looked at the astrocyte-specific ORAI1 knockout mice, we found that they were protected. This effect of astrocyte ORAI1 was specific to motivational behavioral responses in mice after the inflammatory stimulus. Other cognitive functions in the astrocyte knockout mice were unaffected.”
More information:
Michaela M. Novakovic et al, Astrocyte reactivity and inflammation-induced depression-like behaviors are regulated by Orai1 calcium channels, Nature Communications (2023). DOI: 10.1038/s41467-023-40968-6
Citation:
Ion channel linked to brain inflammation identified (2023, September 15)
retrieved 15 September 2023
from https://medicalxpress.com/news/2023-09-ion-channel-linked-brain-inflammation.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.
>>> Read full article>>>
Copyright for syndicated content belongs to the linked Source : Medical Xpress – https://medicalxpress.com/news/2023-09-ion-channel-linked-brain-inflammation.html
