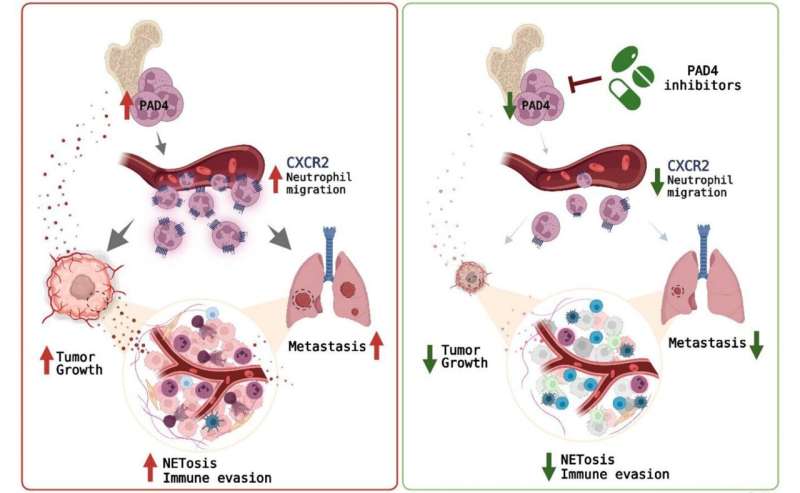
Mechanisms involved in regulation of tumor progression by neutrophil PAD4. Credit: Oncotarget (2023). DOI: 10.18632/oncotarget.28369
A new editorial paper titled “Neutrophil PAD4: how does it function in cancer beyond promoting NETosis?” has been published in Oncotarget.
Expansion of pathologically activated immune suppressive myeloid cells called myeloid-derived suppressor cells (MDSC) is one of the hallmarks of cancer. In most tumor types, MDSC are represented primarily by pathologically activated neutrophils (PMN-MDSC). In this new editorial, researchers Laura Garcia-Gerique and Yulia Nefedova from the Wistar Institute discuss their team’s recent study, published in Cancer Research, identifying a novel mechanism by which neutrophil PAD4 promotes cancer progression.
“Using several transplantable and genetically engineered mouse models, we demonstrated that tumor growth was accompanied by significantly elevated enzymatic activity of neutrophil PAD4. To further clarify the role of PAD4 in tumor progression, we utilized PAD4fl/fl MRP8Cre mice with targeted deletion of PAD4 in myeloid cells, primarily neutrophils,” the researchers explain.
PMN-MDSC originate in the bone marrow and migrate to various sites including tumor tissues and premetastatic niches. These cells have a relatively short lifespan (less than 48 hours), and therefore, are continually replaced from the bone marrow. Tumor-infiltrating PMN-MDSC possess a potent suppressive activity, as they are able to inhibit both antigen-specific immune responses of T cells and non-specific anti-CD3/CD28-stimulated responses. As a result, a highly immunosuppressive environment is created in tumors, which prevents their rejection via immunological mechanisms.
In addition, PMN-MDSC employ non-immunological mechanisms to facilitate tumor progression, including angiogenesis, remodeling of extracellular matrix, and production of cytokines. In patients with solid tumors, levels of MDSC in circulation and tumor tissues have been positively associated with a poor response to the therapy in many types of cancer and represent an independent indicator of poor outcomes. However, many of the details about how PMN-MDSC support cancer progression, and thus approaches for therapeutically targeting these cells remain enigmatic.
“Taken together, our study identified a new mechanism responsible for transcriptional regulation of neutrophil migration and a new mechanism by which neutrophil PAD4 is contributing to tumor progression. PAD4 inhibitors are in development and may enter early phase clinical trials in the future,” the researchers conclude.
More information:
Laura Garcia-Gerique et al, Neutrophil PAD4: how does it function in cancer beyond promoting NETosis?, Oncotarget (2023). DOI: 10.18632/oncotarget.28369
Hui Deng et al, A Novel Selective Inhibitor JBI-589 Targets PAD4-Mediated Neutrophil Migration to Suppress Tumor Progression, Cancer Research (2022). DOI: 10.1158/0008-5472.CAN-21-4045
Provided by
Impact Journals LLC
Citation:
Neutrophil PAD4: How does it function in cancer beyond promoting NETosis? (2023, October 30)
retrieved 30 October 2023
from https://medicalxpress.com/news/2023-10-neutrophil-pad4-function-cancer-netosis.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.
>>> Read full article>>>
Copyright for syndicated content belongs to the linked Source : Medical Xpress – https://medicalxpress.com/news/2023-10-neutrophil-pad4-function-cancer-netosis.html
