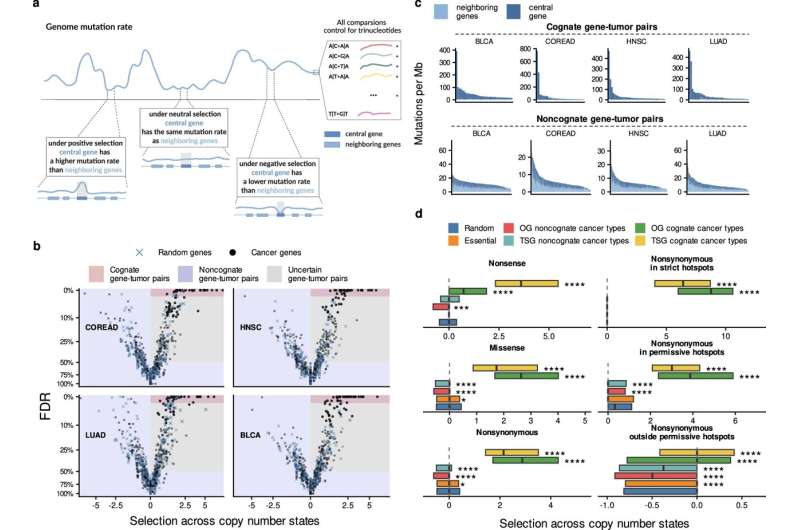
Estimates of somatic selection in various driver gene groups, within and outside the known positively selected hotspot loci. Credit: Nature Communications (2024). DOI: 10.1038/s41467-024-50552-1
Cancer is caused by genetic changes that occur in our cells over time. There are two main types of changes, namely somatic mutations, which are alterations in the DNA sequence, and copy number alterations, which are changes in the number of copies of a particular gene. Until now, these two types of alterations were appreciated to work together in causing cancer, but systematic studies of how they affect the same gene in the same individual were needed.
Scientists at IRB Barcelona have published new findings in Nature Communications revealing that cancer progression is significantly influenced by the interaction between gene copy number alterations and mutations. The research confirms previous knowledge that a decreased copy number correlates with mutations in tumor suppressor genes (which hinders their cancer-protective functions), while an increased copy number correlates with a higher number of oncogene mutations that drive cancer development.
Unexpectedly, the researchers have also established a link between a gain in gene copy number and mutations in tumor suppressor genes, and a link between a lower copy number and more mutations in oncogenes. Both of these paradoxical associations between mutations and copy number were also found to be prevalent in cancer genomes.
Researchers Dr. Elizaveta Besedina and Dr. Fran Supek have provided new insights into the roles of genetic alterations in cancer development, opening new avenues for considering tumor suppressor genes as potential targets for cancer treatment.
“Our research reveals that both increases and decreases in the number of gene copies can occur in surprising ways, which drive cancer evolution,” explains Dr. Supek, head of the Genome Data Science lab at IRB Barcelona, and a professor at the Biotech Research & Innovation Centre at the University of Copenhagen.
“This finding challenges the traditional view of how these interactions occur, and suggests that we may have missed important cancer-causing events in the past,” he adds.
Second-hit events: A common feature of cancer
“Second-hit” events involve one type of genetic alteration, such as a mutation, occurring in combination with another event, commonly a copy number alteration. This combination amplifies the effects of the single events and increases the likelihood of cancer development. This is well-known to occur with inherited cancer-risk variants, such as pathogenic variants in the BRCA1 gene, causing breast and ovarian cancer.
Here, the researchers focused on less studied “second hits” involving somatic mutations. The discovery that these events are common drivers across different types of cancer underscores the importance of considering the combination of different mutation types in genomic analyses.
The scientists developed a novel method called MutMatch to study the combined effects of mutations and copy number alterations in cancer. They analyzed genetic data from around 18,000 tumors to uncover patterns of genetic changes.
“These two-hit events are crucial for understanding the later stages of tumor progression. They set complex interactions between different genetic changes and how they collectively contribute to cancer,” says Dr. Besedina, first author of the study.
Pathways to new therapeutic approaches
This research provides a roadmap for future research into cancer genetics. By exploring the interactions between different types of genetic changes, scientists can gain a deeper understanding of how cancer develops and progresses. This knowledge could lead to the development of new, more effective treatments for the disease.
Understanding the interaction between mutations and copy number alterations enables scientists to identify new targets for cancer therapies, including targets previously overlooked, such as tumor suppressor genes. Because these genes were shown to, paradoxically, drive cancer also by copy number gains, this suggests that many mutations in them are so-called “dominant negative” mutations.
These are, in principle, targetable by therapies. This is how these new genetic insights could lead to the identification of new cancer drugs or repurposing of existing ones, potentially enhancing their efficacy.
Finally, knowledge of the diverse genetic landscapes of tumors can help stratify patients into more precise subgroups and thus contribute to guiding personalized treatment approaches and potentially identifying additional patient groups that could benefit from current therapies.
More information:
Elizaveta Besedina et al, Copy number losses of oncogenes and gains of tumor suppressor genes generate common driver mutations, Nature Communications (2024). DOI: 10.1038/s41467-024-50552-1
Citation:
New patterns in cancer genetics point at tumor suppressor genes as potential therapeutic targets (2024, July 23)
retrieved 23 July 2024
from https://medicalxpress.com/news/2024-07-patterns-cancer-genetics-tumor-suppressor.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.
>>> Read full article>>>
Copyright for syndicated content belongs to the linked Source : Medical Xpress – https://medicalxpress.com/news/2024-07-patterns-cancer-genetics-tumor-suppressor.html










