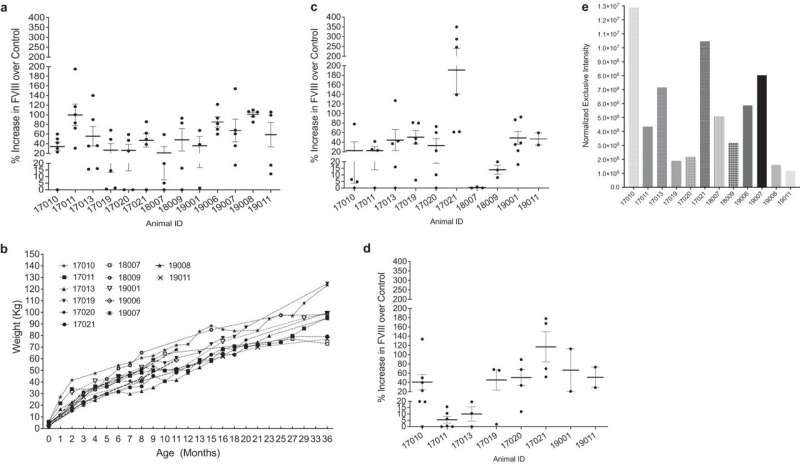
Prenatal transplantation of human PLC-mcoET3 results in increased FVIII activity levels in circulation for at least 3 years after treatment. Credit: Nature Communications (2023). DOI: 10.1038/s41467-023-39986-1
Researchers at the Wake Forest Institute for Regenerative Medicine (WFIRM) have recently published an article in Nature Communications that demonstrates the potential of bioengineered human placental cells as a cure for Hemophilia A.
The article, titled “Transplanting Factor 8/ET3-secreting cells in fetal sheep increases Factor 8 levels long-term without inducing immunity or toxicity,” demonstrates in a preclinical model that transplantation of placental stem cells before birth results in the presence of the missing coagulation factor that would be curative, and that are sustained for at least the first three years of life.
Hemophilia A, the most common inherited bleeding disorder, affects over half a million individuals worldwide, leading to spontaneous, debilitating, and life-threatening bleeding. Although significant advancements have been made, the development of antibodies to current treatments and breakthrough bleeds continues to pose severe challenges.
The study explores the feasibility and safety of prenatal treatment using in-utero transplantation for hemophilia A. Notably, approximately 70% of individuals with hemophilia A have a family history of the disorder, which provides an opportunity for prenatal diagnosis and intervention. This innovative approach aims to provide curative Factor 8 levels, convert severe bleeding disorders to milder forms, and induce immune tolerance, thereby eliminating the risk of inhibitor formation.
“The overall burden of disease in persons with hemophilia continues to be high. A therapeutic option that is curative, or can permanently convert a severe, life-threatening bleeding disorder to a mild issue, would be truly life-changing for Hemophilia A patients and their families,” explained Graca Almeida-Porada, M.D., Ph.D., primary investigator on the study.
“We hope our work will pave the way for the cure of hemophilia A prior to birth, thus enabling the birth of healthy infants who would otherwise have been affected by this disease.”
“These studies, that were possible due to the productive collaboration of multiple investigators, show that prenatal treatment of hemophilia A is feasible and safe,” said Anthony Atala, M.D., director of the Wake Forest Institute for Regenerative Medicine and author on the study.
In this pioneering research, human placental cells were bioengineered to produce Factor 8 coagulation protein and were then transplanted into a preclinical sheep model at a gestational age equivalent to 16–18 weeks in humans.
The results have shown a substantial elevation in Factor 8 plasma levels (>48% above non-transplanted controls) that persisted for the entire 3 years of follow-up. Most importantly, the cells successfully engrafted into major organs, and none of the recipients exhibited any immune responses to either the transplanted cells or the Factor 8 protein they produced.
More information:
Martin Rodriguez et al, Transplanting FVIII/ET3-secreting cells in fetal sheep increases FVIII levels long-term without inducing immunity or toxicity, Nature Communications (2023). DOI: 10.1038/s41467-023-39986-1
Citation:
Prenatal stem cells treat hemophilia A in preclinical study (2023, November 30)
retrieved 1 December 2023
from https://medicalxpress.com/news/2023-11-prenatal-stem-cells-hemophilia-preclinical.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.
>>> Read full article>>>
Copyright for syndicated content belongs to the linked Source : Medical Xpress – https://medicalxpress.com/news/2023-11-prenatal-stem-cells-hemophilia-preclinical.html
