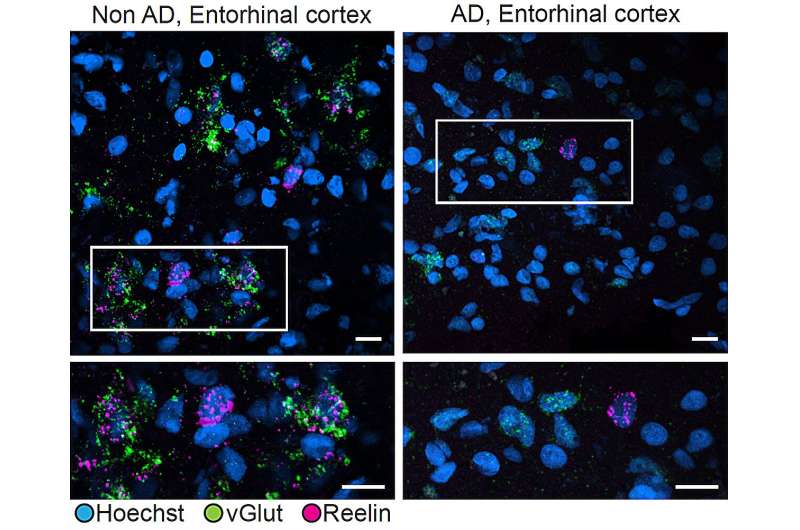
Researchers compared Reelin expression in excitatory neurons in the entorhinal cortex of people with (right) or without (left) Alzheimer’s disease. In people without the disease, vGlut (green), a marker of excitatory neurons, and Reelin (magenta) were often expressed together. In people with Alzheimer’s, excitatory cells exhibited much less Reelin expression. Credit: Tsai Lab/ MIT Picower Institute
An MIT study published in Nature provides new evidence for how specific cells and circuits become vulnerable in Alzheimer’s disease, and hones in on other factors that may help some people show resilience to cognitive decline, even amid clear signs of disease pathology.
To highlight potential targets for interventions to sustain cognition and memory, the authors engaged in a novel comparison of gene expression across multiple brain regions in people with or without Alzheimer’s disease, and conducted lab experiments to test and validate their major findings.
Brain cells all have the same DNA but what makes them differ, both in their identity and their activity, are their patterns of how they express those genes. The new analysis measured gene expression differences in more than 1.3 million cells of more than 70 cell types in six brain regions from 48 tissue donors, 26 of whom died with an Alzheimer’s diagnosis and 22 of whom without.
As such, the study provides a uniquely large, far-ranging and yet detailed accounting of how brain cell activity differs amid Alzheimer’s disease by cell type, by brain region, by disease pathology, and by each person’s cognitive assessment while still alive.
“Specific brain regions are vulnerable in Alzheimer’s and there is an important need to understand how these regions or particular cell types are vulnerable,” said co-senior author Li-Huei Tsai, Picower Professor of Neuroscience and director of The Picower Institute for Learning and Memory and the Aging Brain Initiative at MIT.
“And the brain is not just neurons. It’s many other cell types. How these cell types may respond differently, depending on where they are, is something fascinating we are only at the beginning of looking at.”
Co-senior author Manolis Kellis, professor of computer science and head of MIT’s Computational Biology Group, likened the technique used to measure gene expression comparisons, single cell RNA profiling, to being a much more advanced “microscope” than the ones that first allowed Alois Alzheimer to characterize the disease’s pathology more than a century ago.
“Where Alzheimer saw amyloid protein plaques and phosphorylated tau tangles in his microscope, our single-cell ‘microscope’ tells us, cell by cell and gene by gene, about thousands of subtle yet important biological changes in response to pathology,” said Kellis.
“Connecting this information with the cognitive state of patients reveals how cellular responses relate with cognitive loss or resilience, and can help propose new ways to treat cognitive loss. Pathology can precede cognitive symptoms by a decade or two before cognitive decline becomes diagnosed. If there’s not much we can do about the pathology at that stage, we can at least try to safeguard the cellular pathways that maintain cognitive function.”
Hansruedi Mathys, a former MIT postdoc in the Tsai Lab, who is now an assistant professor at the University of Pittsburgh, Carles Boix, a former graduate student in Kellis’s lab who is now a postdoc at Harvard Medical School, and Leyla Akay, a graduate student in Tsai’s lab, led the study analyzing the prefrontal cortex, entorhinal cortex, hippocampus, anterior thalamus, angular gyrus, and the midtemporal cortex.
The brain samples came from the Religious Order Study and the Rush Memory and Aging Project at Rush University.
Neural vulnerability and Reelin
Some of the earliest signs of amyloid pathology and neuron loss in Alzheimer’s occur in memory-focused regions called the hippocampus and the entorhinal cortex. In those regions, and in other parts of the cerebral cortex, the researchers were able to pinpoint a potential reason why.
One type of excitatory neuron in the hippocampus and four in the entorhinal cortex were significantly less abundant in people with Alzheimer’s than in people without.
Individuals with depletion of those cells performed significantly worse on cognitive assessments. Moreover, many vulnerable neurons were interconnected in a common neuronal circuit. And just as importantly, several either directly expressed a protein called Reelin, or were directly affected by Reelin signaling.
In all, therefore, the findings distinctly highlight especially vulnerable neurons, whose loss is associated with reduced cognition, that share a neuronal circuit and a molecular pathway.
Tsai noted that Reelin has become prominent in Alzheimer’s research because of a recent study of a man in Colombia. He had a rare mutation in the Reelin gene that caused the protein to be more active, and was able to stay cognitively healthy at an advanced age despite having a strong family predisposition to early-onset Alzheimer’s.
The new study shows that loss of Reelin-producing neurons is associated with cognitive decline. Taken together, it may mean that the brain benefits from Reelin, but that neurons that produce it may be lost in at least some Alzheimer’s patients.
“We can think of Reelin as having maybe some kind of protective or beneficial effect,” Akay said. “But we don’t yet know what it does or how it could confer resilience.”
In further analysis, the researchers also found that specifically vulnerable inhibitory neuron subtypes identified in a previous study from this group in the prefrontal cortex were also involved in reelin signaling, further reinforcing the significance of the molecule and its signaling pathway.
To further check their results, the team directly examined the human brain tissue samples and the brains of two kinds of Alzheimer’s model mice. Sure enough, those experiments also showed a reduction in Reelin-positive neurons in the human and mouse entorhinal cortex.
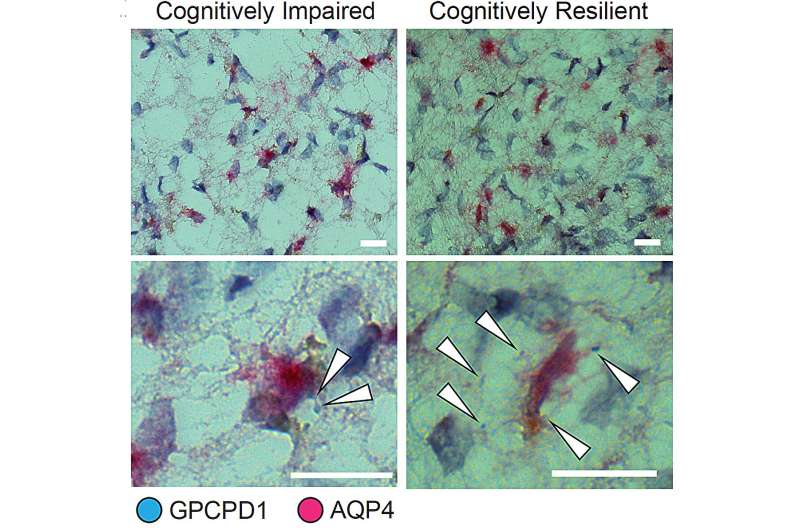
Expression of the gene GPCPD1 in astrocyte cells is associated with cognitive resilience in people with Alzheimer’s pathology. Here white arrows indicate instances of GPCPD1 expression (blue) in astrocyte cells (denoted by AQP4 staining in magenta). There is much more expression in tissue from the cognitively resilient person (right). Credit: Tsai Lab/MIT PIcower Institute
Resilience associated with choline metabolism in astrocytes
To find factors that might preserve cognition, even amid pathology, the team examined which genes, in which cells, and in which regions, were most closely associated with cognitive resilience, which they defined as residual cognitive function, above the typical cognitive loss expected given the observed pathology.
Their analysis yielded a surprising and specific answer: across several brain regions, astrocytes that expressed genes associated with antioxidant activity and with choline metabolism and polyamine biosynthesis were significantly associated with sustained cognition, even amid high levels of tau and amyloid.
The results reinforced previous research findings led by Tsai and Susan Lundqvist in which they showed that dietary supplement of choline helped astrocytes cope with the dysregulation of lipids caused by the most significant Alzheimer’s risk gene, the APOE4 variant.
The antioxidant findings also pointed to a molecule that can be found as a dietary supplement, spermidine, which may have anti-inflammatory properties, although such an association would need further work to be established causally.
As before, the team went beyond the predictions from the single-cell RNA expression analysis to make direct observations in the brain tissue of samples. Those that came from cognitively resilient individuals indeed showed increased expression of several of the astrocyte-expressed genes predicted to be associated with cognitive resilience.
New analysis method, open dataset
To analyze the mountains of single-cell data, the researchers developed a new robust methodology based on groups of coordinately-expressed genes (known as “gene modules”), thus exploiting the expression correlation patterns between functionally-related genes in the same module.
“In principle, the 1.3 million cells we surveyed could use their 20,000 genes in an astronomical number of different combinations,” explains Kellis. “In practice, however, we observe a much smaller subset of coordinated changes. Recognizing these coordinated patterns allows us to infer much more robust changes, because they are based on multiple genes in the same functionally-connected module.”
He offered this analogy: With many joints in their bodies, people could move in all kinds of crazy ways, but in practice they engage in many fewer coordinated movements, like walking, running, or dancing. The new method enables scientists to identify such coordinated gene expression programs as a group.
While Kellis and Tsai’s labs already reported several noteworthy findings from the dataset, the researchers expect that many more possibly significant discoveries still wait to be found in the trove of data. To facilitate such discovery, the team posted handy analytical and visualization tools along with the data on Kellis’s website.
“The dataset is so immensely rich. We focused on only a few aspects that are salient that we believe are very, very interesting, but by no means have we exhausted what can be learned with this dataset,” Kellis said. “We expect many more discoveries ahead, and we hope that young researchers (of all ages) will dive right in and surprise us with many more insights.”
Going forward, Kellis said, the researchers are studying the control circuitry associated with the differentially expressed genes, to understand the genetic variants, the regulators, and other driver factors that can be modulated to reverse disease circuitry across brain regions, cell types, and different stages of the disease.
More information:
Manolis Kellis, Single-cell multiregion dissection of Alzheimer’s disease, Nature (2024). DOI: 10.1038/s41586-024-07606-7. www.nature.com/articles/s41586-024-07606-7
This story is republished courtesy of MIT News (web.mit.edu/newsoffice/), a popular site that covers news about MIT research, innovation and teaching.
Citation:
Study across multiple brain regions discerns Alzheimer’s vulnerability and resilience factors (2024, July 24)
retrieved 24 July 2024
from https://medicalxpress.com/news/2024-07-multiple-brain-regions-discerns-alzheimer.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.
>>> Read full article>>>
Copyright for syndicated content belongs to the linked Source : Medical Xpress – https://medicalxpress.com/news/2024-07-multiple-brain-regions-discerns-alzheimer.html