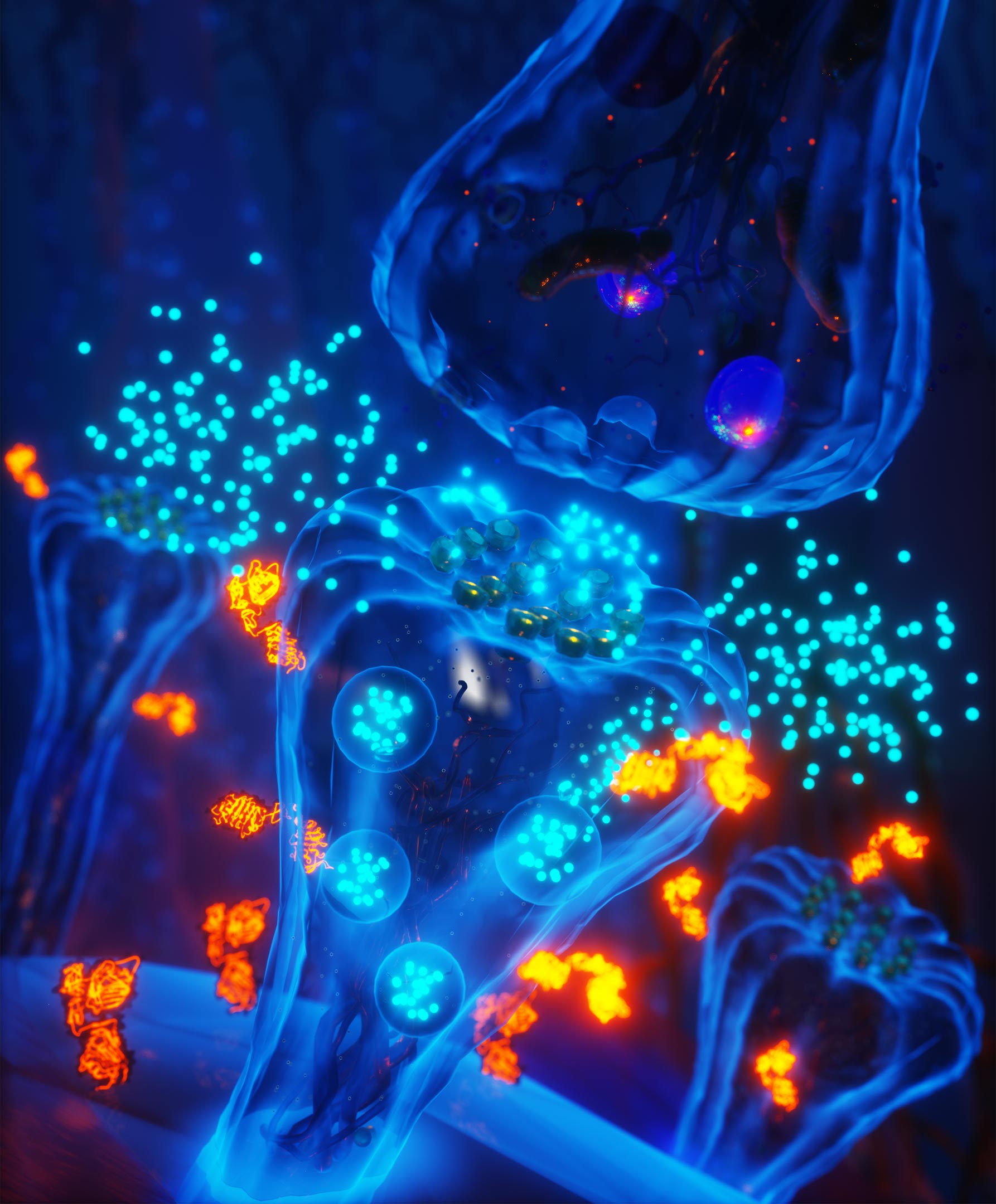
Insulin-like growth factors (IGF1/IGF2-cyan spheres) are released from the postsynaptic compartment of a synapse during plasticity. To understand how IGF1 and IGF2 promote memory formation, Tu et al. developed a biosensor to detect the activity of the IGF1-receptor during synaptic plasticity, the cellular process that strengthens connections between neurons during learning. They discovered a region-specific, autocrine signaling mechanism in the hippocampus that promotes synapse growth and strength. Disrupting IGF signaling impaired plasticity, highlighting the critical role of the insulin superfamily in maintaining cognitive health. Credit: Illustration by Ella Maru Studios
Scientists at the Max Planck Institute discovered a mechanism where the insulin superfamily of hormones, specifically IGF1 and IGF2, are locally produced and released by neurons during synaptic plasticity, promoting memory formation and cognitive health. This breakthrough could potentially guide future research in combating cognitive decline and Alzheimer’s disease.
The insulin superfamily of hormones, which includes insulin, insulin-like growth factor 1 (IGF1), and insulin-like growth factor 2 (IGF2), has a crucial role in not only regulating blood sugar, metabolism, and growth, but also in promoting healthy brain development and function, particularly learning and memory.
These hormones can enter the brain from the liver through the bloodstream or be synthesized directly in neurons and glial cells within the brain. They bind to receptors such as the IGF1-Receptor, activating signals that modulate neuron growth and activity. A disruption in this signaling pathway can contribute to cognitive decline and diseases such as Alzheimer’s.
Investigating IGF1 and IGF2 in Brain Health
In an effort to understand how IGF1 and IGF2 promote brain health, scientists explored the activation of this signaling pathway in the hippocampus, a region of the brain crucial for learning and memory. More specifically, they sought to determine whether IGF signaling was active during synaptic plasticity, the cellular process that strengthens connections between neurons during memory formation and guards against cognitive decline.
Using a Biosensor to Detect IGF1-Receptor Activity
To facilitate this, scientists from the Max Planck Institute developed a biosensor to detect when the IGF1-Receptor was active. This allowed them to visualize the activity of the signaling pathway involved in plasticity. When a synapse was undergoing plasticity, the scientists observed that the IGF1-Receptor was robustly activated in the strengthening synapse and nearby synapses. This receptor activation was critical for synaptic growth and strengthening during plasticity. However, where the IGF that activates the receptor was coming from was unknown.
However, Dr. Xun Tu, lead researcher and first author of the scientific publication, described how being able to visualize the receptor activation during plasticity gave them a clue. “The fact that the activation of the IGF-Receptor was localized near the synapse undergoing plasticity suggested that IGF1 or IGF2 might be produced in hippocampal neurons and locally released during plasticity,” she explained.
Exploring the Production and Release of IGF1 and IGF2
To investigate this hypothesis, the scientists tested whether IGF1 and IGF2 were produced and could be released from hippocampal neurons. Interestingly, they discovered a region-specific difference in the production of IGF1 and IGF2. One group of neurons in the hippocampus, CA1 neurons, produced IGF1; another group, CA3 neurons, produced IGF2. When either CA1 or CA3 neurons were activated in a way that mimicked synaptic plasticity, IGF was released. Importantly, when the scientists disrupted the ability of the neurons to produce IGF, the activation of the IGF1-Receptor during plasticity and synaptic growth and strengthening was blocked.
Significance of the Findings
Dr. Ryohei Yasuda, senior author on the publication and Max Planck Scientific Director, summarized the findings. “This work reveals a local, autocrine mechanism in neurons that is critical for brain plasticity. When a synapse undergoes plasticity, IGF is released locally to activate the IGF1-Receptor on the same neuron. Disrupting this mechanism impairs the plasticity, highlighting its critical role in maintaining cognitive health.”
Implications for Future Research
This discovery of this new mechanism sheds light on how memories are encoded in the brain and highlights the importance of further study on the insulin superfamily of hormones in the brain. The scientists hope that understanding the mechanism through which IGF hormones facilitate brain plasticity, will lead to research into whether targeting this signaling pathway could prevent cognitive decline and combat diseases like Alzheimer’s.
Reference: “Local autocrine plasticity signaling in single dendritic spines by insulin-like growth factors” by Xun Tu, Anant Jain, Paula Parra Bueno, Helena Decker, Xiaodan Liu and Ryohei Yasuda, 2 August 2023, Science Advances.
DOI: 10.1126/sciadv.adg0666
This research was supported by the Louis D Srybnik Foundation Inc. and Foundation for the Art, Science, and Education Inc., the National Institutes of Health (Grant Numbers: R35NS116804, DP1NS096787, and R01MH080047), and the Max Planck Florida Institute for Neuroscience. This content is solely the responsibility of the authors and does not necessarily represent the official views of the funders.
>>> Read full article>>>
Copyright for syndicated content belongs to the linked Source : SciTechDaily – https://scitechdaily.com/biosensor-vision-revealing-the-crucial-role-of-insulin-like-hormones-in-brain-plasticity/










