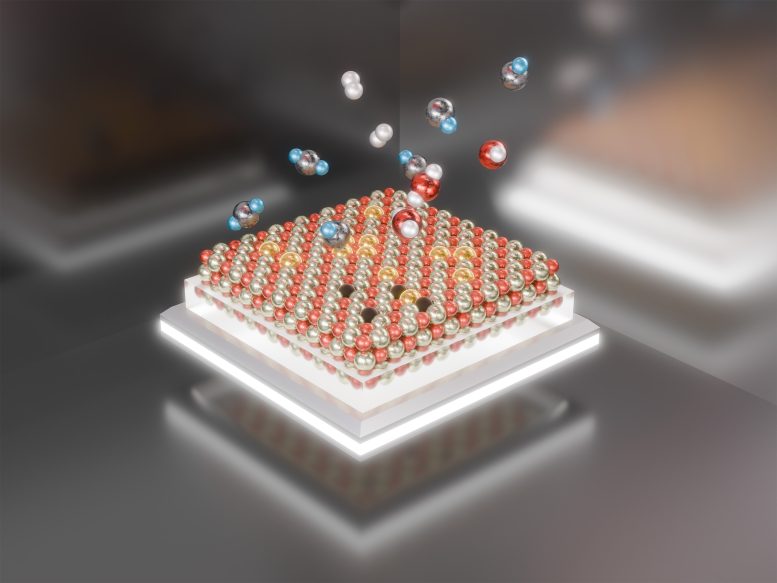
Scientists have shown that platinum atoms (gold spheres) on cerium oxide (red and silver/black surface) can assemble into active nanocatalysts under reaction conditions and then disassemble when cooled down before reuse. Credit: Valerie Lentz/Brookhaven National Laboratory
Combination of techniques reveals how a nanoparticle catalyst forms from individual atoms during operation and subsequently breaks down for recycling.
Researchers at the U.S. Department of Energy’s Brookhaven National Laboratory and Stony Brook University, along with their partners, have made significant discoveries regarding the reversible assembly and disassembly of a platinum catalyst. This new insight could provide information about the catalyst’s stability and potential for reuse. Their recent study, published in the journal Nanoscale, explores how individual platinum atoms on a cerium oxide base come together to form active catalytic nanoparticles during reactions. Intriguingly, these particles then break apart when the reaction ceases.
Fragmentation may sound shattering, but the scientists say it could be a plus.
“Such reversible fragmentation of a platinum nanocatalyst on cerium oxide could be potentially useful for controlling the catalyst’s long-term stability,” said Anatoly Frenkel, a chemist at Brookhaven Lab and professor at SBU who led the research.
When the platinum atoms return to their starting positions, they can be used again to remake active catalytic particles. Plus, the post-reaction fragmentation makes those active particles much less likely to fuse together irreversibly, which is a common mechanism that ultimately deactivates many nanoparticle catalysts.
“Part of the definition of a catalyst is that it helps disassemble and reassemble reacting molecules to form new products,” Frenkel noted. “But it was shocking to see a catalyst that also assembles and disassembles itself in the process.”
Assembly/disassembly
The paper describes how the scientists observed the nanoparticles forming as single platinum atoms aggregated on the cerium oxide surface at 572 degrees Fahrenheit (300 degrees Celsius) — the temperature of the reaction they were studying.
“After the reaction, we expected that these nanoparticles would stabilize once back at room temperature in whatever particle size they reached when they were activated,” Frenkel said. “But what we observed was a reverse process. The particles began fragmenting into single atoms again.”
A schematic showing how single platinum atoms (Pt SA) on a cerium oxide surface self-assemble at elevated temperature (T) to become active nanocatalyst (NC) particles. At high temperature, the catalyst converts hydrogen (H2) and carbon dioxide (CO2) into water (H2O) and carbon monoxide (CO). Upon cooling, the nanocatalyst particles disassemble into single platinum atoms that can be reactivated and used again. Credit: Brookhaven National Laboratory
The team had a hypothesis to explain what they were seeing, which was confirmed by thermodynamic calculations performed by theory colleagues at Chungnam National University in Korea. Carbon monoxide, one of the products of the reaction — often considered a “poison” for catalysts — was actively tearing the nanoparticles apart.
“Carbon monoxide molecules have a very strong repulsive interaction when they are next to each other,” Frenkel explained. During the “reverse water gas shift” reaction, which converts carbon dioxide (CO2) and hydrogen (H2) into carbon monoxide (CO) and water (H2O) at high temperatures, the CO typically leaves the catalyst surface as a gas. But once the heat is turned off, the CO molecules bind strongly to the platinum atoms of the catalyst. This brings the CO molecules closer to each other as the system cools down and their numbers rise.
“That is a perfect storm,” said Frenkel.
“When the CO molecules find themselves very close together on the surface of the nanoparticles, they repel. And, when they repel, because they are strongly bound to the platinum atoms, they sort of pull the least-tightly bound platinum atoms from the perimeter of the nanoparticle and drag them onto cerium oxide support,” Frenkel said.
Multimodal imaging
The scientists used a combination of atomic-level spectroscopic and imaging techniques to make these observations.
One technique used bright X-rays at the Quick x-ray Absorption and Scattering beamline of the National Synchrotron Light Source-II (NSLS-II) to produce a spectrum of the energy absorbed by the atoms that make up the catalyst. The scientists used this technique to study the catalyst at different temperatures and stages of the reaction. These X-ray absorption spectra are strongly influenced by the electronic states of the atoms and can be used to decipher which atoms are nearby.
“This technique can tell us that the platinum atoms have oxygen neighbors from the cerium oxide particles of the catalyst support, carbon monoxide neighbors from the reaction products, or other metal neighbors — more platinum atoms,” Frenkel said. But it “lumps together information from many platinum atoms and only gives average information,” he noted.
“It can’t tell us whether all platinum atoms have the same environment or whether we have different groups of atoms — some dispersed on the support and some within the nanoparticles. We needed additional tools to unravel the possibilities,” he said.
Infrared spectroscopy, performed in Frenkel’s Structure and Dynamics of Applied Nanomaterials (SDAN) laboratory in the Brookhaven Lab Chemistry Division, revealed the presence of two distinct groups —single atoms with no metal neighbors and nanoparticles made only of platinum. The scientists used the technique to track the relative abundance of each group as the reaction progressed.
“This technique tells us how molecules such as CO interact with our platinum atoms. Do they show features of single atoms only or nanoparticles only or both?” Frenkel said. “During the cooling down after the reaction, we observed that CO was interacting with single atoms again.”
Electron microscopy, performed by Lihua Zhang of Brookhaven’s Center for Functional Nanomaterials (CFN), produced nanoscale images of both species — single atoms and nanoparticles. These images show that, at room temperature before the catalyst is activated, there are no nanoparticles, and after the reaction, “we saw both nanoparticles and single atoms,” Frenkel said.
“These techniques together tell us that, once the reaction stops and the temperature drops, the nanoparticles have started to fragment into single atoms,” Frenkel said. “Each measurement independently would not have given us enough data to understand what we are dealing with. We couldn’t have done this work without our collaborators at NSLS-II and CFN and without the capabilities at these DOE Office of Science user facilities.”
Change and disorder
Understanding these differences at stages of the reaction is critical to understanding how the catalyst works, Frenkel said.
“In our experiment, we deliberately went from one extreme to the other. We went from only single atoms to only nanoparticles. In the process, we had them coexist at different fractions so we could systematically investigate how the catalytic activity changes, how the structure changes,” he said.
Frenkel noted that the nanoparticles don’t assemble perfectly. They have more defects — irregular atomic sites — compared to nanoparticles synthesized by commonly used methods. These defects could turn out to be another feature that improves catalytic performance. That’s because disorder, or strain, can contribute to the alignment of the electronic levels of chemical reactants and metal atoms in the catalyst so they can interact more easily, he explained.
“People try to design catalysts with these types of imperfections deliberately; our method incorporates strain naturally,” he said.
In addition, due to these relatively disordered structures, nanoparticles assembled from single atoms might not be as tightly bound as a perfect array of atoms would be. That could make it easier for them to disassemble for reuse when the reaction turns off.
Reference: “Unravelling the origin of reaction-driven aggregation and fragmentation of atomically dispersed Pt catalyst on ceria support” by Haodong Wang, Hyuk Choi, Ryuichi Shimogawa, Yuanyuan Li, Lihua Zhang, Hyun You Kim and Anatoly I. Frenkel, 15 May 2024, Nanoscale.
DOI: 10.1039/D4NR01396D
This work was funded by the DOE Office of Science and by the National Research Foundation of Korea. In addition to making use of capabilities at NSLS-II and CFN, the scientists used computing resources at the Scientific Data and Computing Center, a component of the Computational Science Initiative at Brookhaven Lab.
>>> Read full article>>>
Copyright for syndicated content belongs to the linked Source : SciTechDaily – https://scitechdaily.com/chemists-discover-how-platinum-catalysts-assemble-and-disassemble-themselves/