The 2023 Nobel Prize in medicine was awarded to Katalin Karikó and Drew Weissman, two of the scientists whose work helped pave the way for mRNA vaccines against COVID-19. Karikó is a biochemist from Sagan’s University in Hungary and an adjunct professor at the University of Pennsylvania. Karikó was also senior vice president and head of RNA protein replacement at BioNTech until 2022 and has been an advisor for the company. Weissman is a vaccine researcher at the University of Pennsylvania’s Perelman School of Medicine and Director of the Penn Institute for RNA Innovations.
[Related: How does an mRNA vaccine work?]
The prize is awarded by the Nobel Assembly of Sweden’s Karolinska Institute medical university and comes with its signature gold medicine and about $1 million (11 million Swedish crowns).
“Through their groundbreaking findings, which have fundamentally changed our understanding of how mRNA interacts with our immune system, the laureates contributed to the unprecedented rate of vaccine development during one of the greatest threats to human health in modern times,” the panel wrote in a press release.
A potential game changer for vaccines
Previously, growing viruses, or at least pieces of viruses, were necessary to make a vaccine. The viruses were often cultivated in giant vats of cells or in or in chicken eggs, like the majority of flu shots. The viruses are then purified before being made into a vaccine.
Using messenger RNA (mRNA) in vaccines is very different. It starts with a snippet of genetic code that brings instructions for making proteins. If the right virus protein is selected for the vaccine, then the body produces its own defenses against the virus.
Genetic information encoded in DNA is transferred to mRNA, which is used as a blueprint for protein production in our cells. During the 1980s, efficient methods for producing mRNA without cell culture began. This process, called in vitro transcription, accelerated the development of molecular biology applications to several fields, but using mRNA technologies for vaccines had several roadblocks. In vitro transcribed mRNA was considered unstable and challenging to deliver since it required scientists to develop sophisticated carrier lipid systems to enclose the mRNA and produced some early inflammatory reactions.
[Related: The FDA just green-lit America’s first COVID vaccine.]
Karikó was devoted to the idea of using mRNA for vaccines and other therapeutics during the 1990s when she became colleagues with Weissman. Weissman was interested in dendritic cells, which are important for immune surveillance and triggering vaccine-induced immune responses.
The breakthrough
The two began to focus on how different RNA types interact with the immune system and noticed that the dendritic cells recognize in vitro transcribed mRNA as a foreign substance. This leads to their activation and release of inflammatory signaling molecules.mRNA from mammalian cells did not give rise to the same reaction, the panel wrote. Different types of mRNA, therefore, must be distinguishable.
RNA contains four bases that are abbreviated A, U, G, and C. These letters correspond to the letters of genetic code in DNA A, T, G, and C. Karikó and Weissman knew that bases in RNA from mammalian cells are often chemically modified, and in vitro transcribed mRNA is not. They then wondered if the absence of altered bases in the in vitro transcribed RNA could explain unwanted inflammatory reactions.
To learn more, they created different variants of mRNA which had unique chemical alterations at their bases. They delivered these to dendritic cells and the results were huge.
The inflammatory response was almost wiped out when these base modifications were included in the mRNA. This was a seismic shift in scientific understanding of how cells recognize and respond to different forms of mRNA. . Their results were published in 2005.
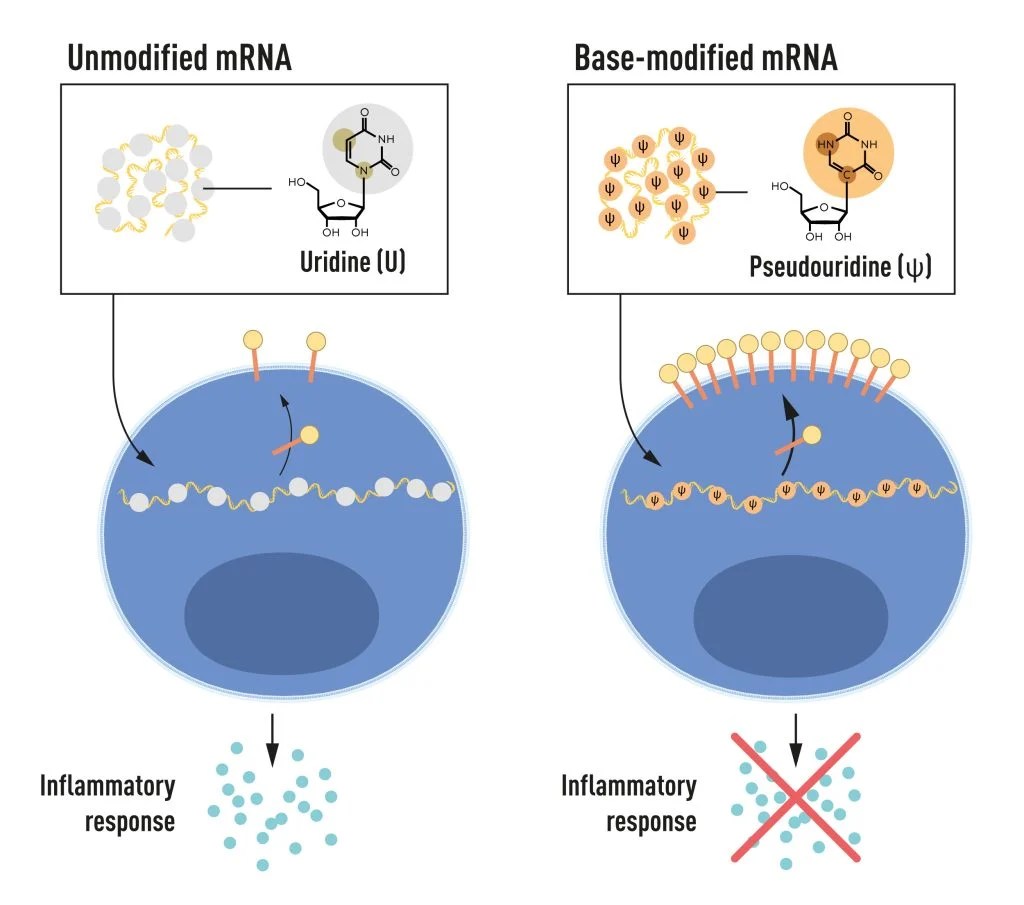 mRNA contains four different bases, abbreviated A, U, G, and C. The Nobel Laureates discovered that base-modified mRNA can be used to block activation of inflammatory reactions (secretion of signaling molecules) and increase protein production when mRNA is delivered to cells. CREDIT: Mattias Karlén/The Nobel Committee for Physiology or Medicine.
mRNA contains four different bases, abbreviated A, U, G, and C. The Nobel Laureates discovered that base-modified mRNA can be used to block activation of inflammatory reactions (secretion of signaling molecules) and increase protein production when mRNA is delivered to cells. CREDIT: Mattias Karlén/The Nobel Committee for Physiology or Medicine.
COVID-19 and The Future
Interest in mRNA technology began to accelerate with their discovery. In 2010 several companies were working on developing the method for viruses such as Zika virus and MERS-CoV.
[Related: White House invests $5 billion in new COVID vaccines and treatments as national emergency ends.]
After the COVID-19 pandemic began, two base-modified mRNA vaccines encoding the SARS-CoV-2 surface protein were developed at a breakneck pace. Two highly effective vaccines were approved in December 2020.
One of the major advantages of mRNA technology was that vaccines could be made in extremely large quantities since their main components are made in laboratories, Exeter University infectious disease expert Bharat Pankhania told the Associated Press. mRNA tech could be used to refine vaccines for diseases including Ebola, malaria, and dengue, as well as help immunize people against auto-immune diseases like lupus and even some types of cancer.
The laureates will receive their awards at ceremonies on December 10. The 2022 medicine prize was awarded to Svante Pääbo for sequencing the genome of the Neanderthal. Other past winners include Karl Landsteiner in 1930 for the discovery of human blood groups and co-winner Alexander Fleming for the discovery of penicillin in 1945.

>>> Read full article>>>
Copyright for syndicated content belongs to the linked Source : Popular Science – https://www.popsci.com/health/nobel-prize-medicine-covid-19-mrna/










