A new antibiotic that works by disrupting two different cellular targets would make it 100 million times more difficult for bacteria to evolve resistance, according to new research from the University of Illinois Chicago.
Antimicrobial resistance (AMR) is one of the top global public health and development threats. It is estimated that bacterial AMR was directly responsible for 1.27 million global deaths in 2019 and contributed to 4.95 million deaths. AMR puts many of the gains of modern medicine at risk. It makes infections harder to treat and makes other medical procedures and treatments – such as surgery, caesarean sections and cancer chemotherapy – much riskier. If this was not solved there would be an antibiotics pipeline and access crisis. There is an inadequate research and development pipeline in the face of rising levels of resistance, and urgent need for additional measures to ensure equitable access to new and existing vaccines, diagnostics and medicines. In addition to death and disability, AMR has significant economic costs. The World Bank estimates that AMR could result in US$ 1 trillion additional healthcare costs by 2050, and US$ 1 trillion to US$ 3.4 trillion gross domestic product (GDP) losses per year by 2030.
“The beauty of this antibiotic is that it kills through two different targets in bacteria,” said Alexander Mankin, distinguished professor of pharmaceutical sciences at UIC. “If the antibiotic hits both targets at the same concentration, then the bacteria lose their ability to become resistant via acquisition of random mutations in any of the two targets.”
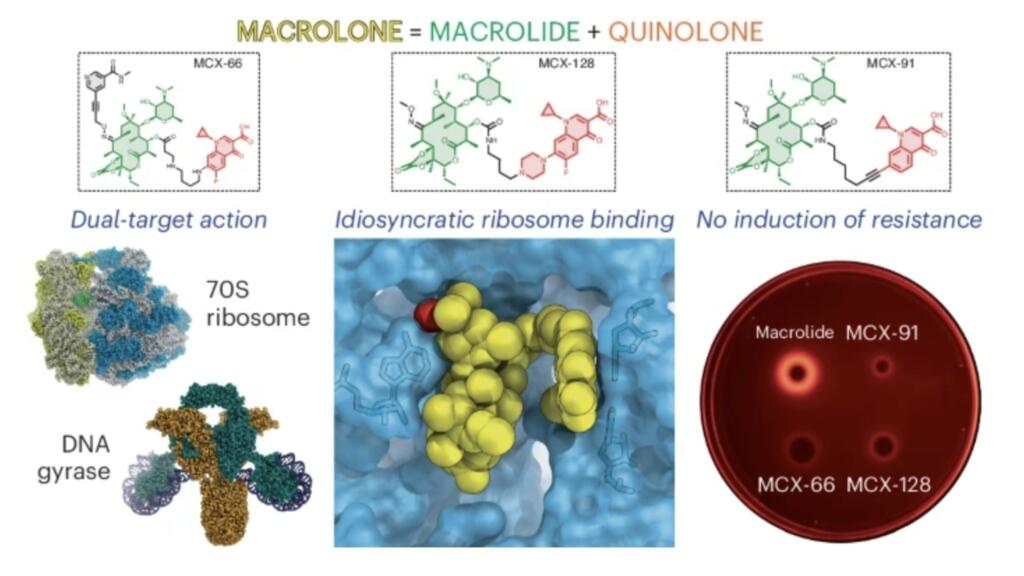
Macrolones are synthetic antibiotics that combine the structures of two widely used antibiotics with different mechanisms. Macrolides, such as erythromycin, block the ribosome, the protein-manufacturing factories of the cell. Fluoroquinolones, such as ciprofloxacin, target a bacteria-specific enzyme called DNA gyrase.
Two UIC laboratories led by Yury Polikanov, associate professor of biological sciences, Mankin and Nora Vázquez-Laslop, research professor of pharmacy, examined the cellular activity of different macrolone drugs.
Polikanov’s group, which specializes in structural biology, studied how these drugs interact with the ribosome and found that they bind more tightly than traditional macrolides. The macrolones were even capable of binding and blocking ribosomes from macrolide-resistant bacterial strains and failed to trigger the activation of resistance genes.
Other experiments tested whether the macrolone drugs preferentially inhibited the ribosome or the DNA gyrase enzymes at various doses. While many designs were better at blocking one target or another, one that interfered with both at its lowest effective dose stood out as the most promising candidate.
Studies show the cost of resistant infection ranges is up to about $30,000 per patient episode (2020 dollars and costs). On average people stay an extra 7.4 days in the hospital and the odds of dying nearly double (1.84 times) and there is about 1.492 increased readmission likelihood.
Abstract
Growing resistance toward ribosome-targeting macrolide antibiotics has limited their clinical utility and urged the search for superior compounds. Macrolones are synthetic macrolide derivatives with a quinolone side chain, structurally similar to DNA topoisomerase-targeting fluoroquinolones. While macrolones show enhanced activity, their modes of action have remained unknown. Here, we present the first structures of ribosome-bound macrolones, showing that the macrolide part occupies the macrolide-binding site in the ribosomal exit tunnel, whereas the quinolone moiety establishes new interactions with the tunnel. Macrolones efficiently inhibit both the ribosome and DNA topoisomerase in vitro. However, in the cell, they target either the ribosome or DNA gyrase or concurrently both of them. In contrast to macrolide or fluoroquinolone antibiotics alone, dual-targeting macrolones are less prone to select resistant bacteria carrying target-site mutations or to activate inducible macrolide resistance genes. Furthermore, because some macrolones engage Erm-modified ribosomes, they retain activity even against strains with constitutive erm resistance genes.
“By basically hitting two targets at the same concentration, the advantage is that you make it almost impossible for the bacteria to easily come up with a simple genetic defense,” Polikanov said.

Brian Wang is a Futurist Thought Leader and a popular Science blogger with 1 million readers per month. His blog Nextbigfuture.com is ranked #1 Science News Blog. It covers many disruptive technology and trends including Space, Robotics, Artificial Intelligence, Medicine, Anti-aging Biotechnology, and Nanotechnology.
Known for identifying cutting edge technologies, he is currently a Co-Founder of a startup and fundraiser for high potential early-stage companies. He is the Head of Research for Allocations for deep technology investments and an Angel Investor at Space Angels.
A frequent speaker at corporations, he has been a TEDx speaker, a Singularity University speaker and guest at numerous interviews for radio and podcasts. He is open to public speaking and advising engagements.
>>> Read full article>>>
Copyright for syndicated content belongs to the linked Source : Next Big Future – https://www.nextbigfuture.com/2024/07/new-antibiotic-could-solve-bacteria-resistance-and-save-5-million-lives-per-year.html










