Organ-on-chip (OOC) systems, which use human cells to create dynamic simulations of organ-level function, tissue structure, and disease phenotypes, are revolutionizing tissue engineering. However, the recapitulation of in vivo-like tissue-tissue cross-talk and morphogenesis is frequently limited by nonbiological components found in OOC devices.
Duke University biomedical engineers have created an ultrathin silk membrane that can be utilized in organ-on-a-chip simulations to resemble the natural surroundings of bodily tissues and cells more closely.
They created a kidney glomerulus-on-a-chip by utilizing human-induced pluripotent stem cells and a biomimetic ultrathin membrane to replicate glomerular morphogenesis and barrier function.
When utilized in a kidney organ-on-a-chip platform, the membrane aided in the growth of tissues to replicate the functions of both healthy and damaged kidneys.
This novel membrane enables researchers to better manage the growth and function of any organ’s primary cells and tissues by allowing the cells to grow closer together. This will enable researchers to test medicines and more accurately replicate various ailments.
These polymer membranes, which range in thickness from 30 to 50 microns, impede cell-to-cell contact and restrict cell proliferation. In contrast, the extracellular membranes found in human organs are frequently less than one micron thick.
Wouldn’t it be wonderful if we could create a protein-based substance that is thin enough to cut and yet replicates the structure of these natural membranes?
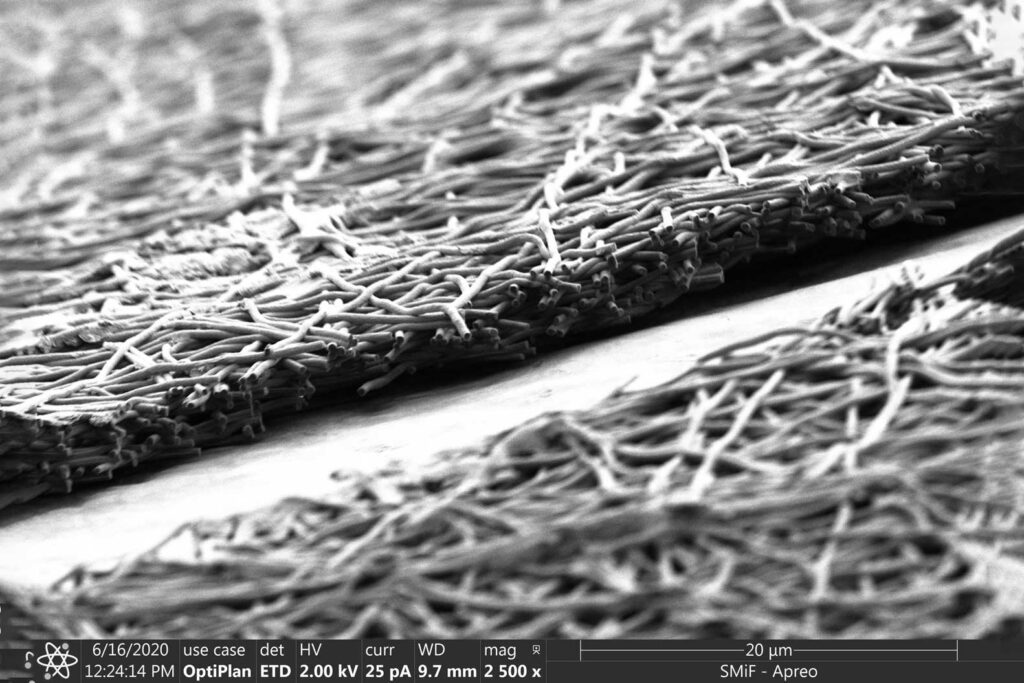
Scientists’ discovery of silk fibroin—a silkworm protein that can be electrically spun into a membrane—was prompted by this question. Under a microscope, silk fibroin resembles spaghetti or a picture by Jackson Pollock. The porous material, composed of long, intertwining fibers, has been utilized to produce scaffolds for applications such as wound healing. Its structure is more similar to the extracellular matrix found in human organs.
Thanks to the silk fibroin, they reduced the membrane’s thickness from 50 to five microns or less, bringing us one step closer to what we would find in a living thing.
Scientists tested this new membrane by applying the material to their kidney chip models.
This OOC platform, which is around the size of a quarter and is constructed of clear plastic, is designed to mimic a cross-section of a human kidney, specifically the glomerular capillary wall, a vital component of the organ that is made up of groups of blood capillaries and is in charge of filtering blood.
After attaching the membrane, the scientists filled the chip with human-induced pluripotent stem cell variants. They noticed that the cells’ ability to communicate signals through the fragile membrane aided their differentiation into podocytes, vascular endothelial cells, and glomerular cells. Additionally, the platform stimulated the growth of endothelial fenestrations or holes that permit fluid to move between the cellular layers in the developing tissue.
After the experiment, these various kidney cell types formed a glomerular capillary wall and effectively filtered molecules according to size.
Musah said, “The new microfluidic chip system’s ability to simulate in vivo-like tissue-tissue interfaces and induce the formation of specialized cells, such as fenestrated endothelium and mature glomerular podocytes from stem cells, holds significant potential for advancing our understanding of human organ development, disease progression, and therapeutic development.”
Musah and associates hope to use this technology to understand the mechanisms underlying kidney illness further as they continue enhancing their model. Though the condition affects about 15% of adult Americans, researchers have not yet developed reliable models. Additionally, patients frequently wait until their kidneys have suffered significant damage before receiving a diagnosis, at which point they often need kidney transplants or dialysis.
According to Mou, scientists may find novel disease biomarkers by using this platform to create models of renal illness. It could also be used to find potential drugs using a variety of renal disease models. The opportunities are pretty thrilling.
Journal Reference:
Xingrui Mou, Jessica Shah, Yasmin Roye, Carolyn Du, Samira Musah. An Ultrathin Membrane Mediates Tissue-Specific Morphogenesis and Barrier Function in a Human Kidney Chip. Science Advances. DOI: 10.1126/sciadv.adn2689
>>> Read full article>>>
Copyright for syndicated content belongs to the linked Source : Tech Explorist – https://www.techexplorist.com/scientists-silkworms-grow-organ-tissues-in-labs/84873/#utm_source=rss&utm_medium=rss&utm_campaign=scientists-silkworms-grow-organ-tissues-in-labs