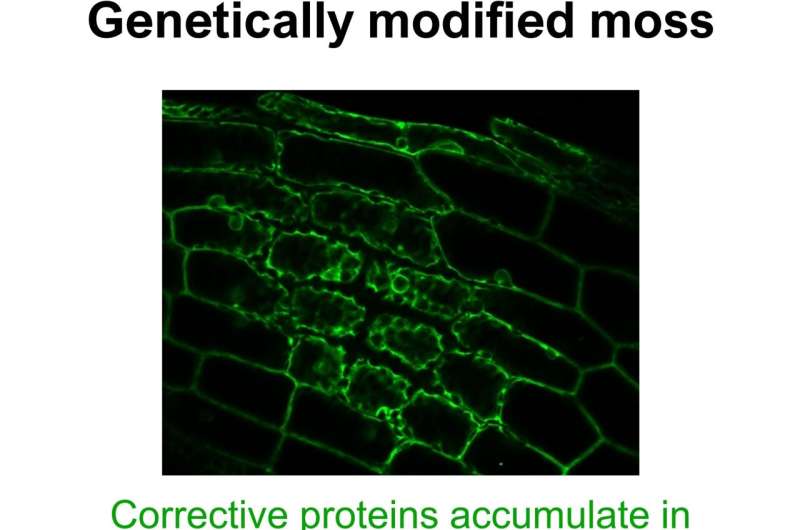
The “Tipp-Ex” proteins in the genetically modified moss accumulated in the cytosol, where they modified copies of genes. This is why the plant usually transports the proteins quickly into its organelles. Credit: Elena Lesch/University of Bonn
Plants have special corrective molecules at their disposal that can make retrospective modifications to copies of genes. However, it would appear that these “Tipp-Ex proteins” do not have permission to work in all areas of the cell, only being used in chloroplasts and mitochondria.
A study by the University of Bonn has now explained why this is the case. It suggests that the correction mechanism would otherwise modify copies that have nothing wrong with them, with fatal consequences for the cell. The findings have been published in The Plant Journal.
Plant cells possess a whole host of specialized structures known as organelles, of which two particularly important ones are the chloroplasts and mitochondria. The former use light energy to convert carbon dioxide and water into oxygen and sugar, while the latter do more or less the same thing in reverse: they “burn” sugar and other compounds to generate the energy needed for numerous cellular processes.
The two organelles are unique in that they have their own genes. This genetic material works like sets of assembly instructions for key molecules that the organelles require for their work. If a chloroplast needs to make a certain protein, for instance, it first orders a copy of the relevant assembly instructions that it can then use to produce the protein.
Genes from chloroplasts and mitochondria often defective
“However, the genes in chloroplasts and mitochondria often contain defects,” explains Elena Lesch, a doctoral student at the University of Bonn’s Institute for Cellular and Molecular Botany. “So the copies have to be corrected, otherwise the proteins assembled based on their instructions won’t work.”
For this, plants use a kind of Tipp-Ex—special molecules that belong to the group of pentatricopeptide repeat (PPR) proteins.
Plants have at least a dozen and, in some cases, as many as several thousand of these special PPR proteins, each one of which corrects highly specific defects. It is as if every word in a newspaper had its own sub-editor. Rather than being made in the organelles in which they are used, however, the PPR proteins are manufactured outside of the organelles, within the cytosol.
The cytosol is also packed full of gene copies, although these come from the cell’s nucleus, where most of the many thousands of the plant’s genes are stored. By contrast, mitochondria and chloroplasts only contain a few dozen genes each. The “Tipp-Ex proteins” could theoretically correct the copies inside the cytosol too. “But they don’t,” Lesch says. “They only do their work in the organelles, and we wanted to know why.”
Swamping the transportation mechanism into the organelles
One reason might be that the “molecular sub-editors” are simply moved too quickly from the cytosol into the organelles. To investigate this possibility, the researchers fitted a kind of molecular switch to PPR genes inside some of the moss Physcomitrium. This enabled them to make the cells produce very large quantities of PPR proteins virtually at the touch of a button.
“We were able to demonstrate that this swamps the transportation mechanism,” reveals Lesch’s colleague Mirjam Thielen, who conducted many of the experiments. “It caused a pile-up of PPR proteins in the cytosol.”
Once they had arrived in the cytosol, they began to modify copies from the nucleus. “We analyzed the changes they made and saw that the proteins had modified a great many sets of assembly instructions that would actually have been correct,” Lesch says.
“Incorrect interventions like these are counterproductive, of course, because they can put protein functions at risk.” But why should this be happening in the first place? As well as detecting defects, the PPR proteins also bind to what are known as off-target sequences, areas that may look like a defective sequence but are actually perfectly fine.
“With copies of tens of thousands of genes jostling for space inside the cytosol, the risk of these off-target sequences being corrected incorrectly would be high,” Lesch notes.
Production of ‘Tipp-Ex’ molecules subject to strict regulation
To prevent this, plants generally only ever make relatively low quantities of PPR proteins, which are then transported straight into the organelles before the molecular “Tipp-Ex” in the cytosol can do any harm. Because the number of genes—and thus how many copies of them there are—inside the chloroplasts and mitochondria is manageable, no such miscorrections tend to occur there.
The study is supplying new insights into how these corrective proteins identify their targets. In the future, therefore, it may be possible to use the findings to make highly targeted modifications to specific copies of genes inside mitochondria and chloroplasts and to investigate the effect of such modifications.
Given the important roles that these organelles play in plants’ energy metabolism, this also opens up scope for some interesting practical applications.
More information:
Mirjam Thielen et al, Conquering new grounds: plant organellar C‐to‐U RNA editing factors can be functional in the plant cytosol, The Plant Journal (2024). DOI: 10.1111/tpj.16804
Citation:
Study shows plants restrict use of corrective ‘Tipp-Ex proteins’ (2024, May 17)
retrieved 17 May 2024
from https://phys.org/news/2024-05-restrict-tipp-proteins.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.
>>> Read full article>>>
Copyright for syndicated content belongs to the linked Source : Phys.org – https://phys.org/news/2024-05-restrict-tipp-proteins.html