Springtime delivers the world’s tiniest zoo babies.
Posted on May 25, 2024 12:00 PM EDT

This noble necklace worm, laden with eggs, will eventually release her developed larvae into the water column as plankton. Photo by the Hakai Institute
This article was originally featured on Hakai Magazine, an online publication about science and society in coastal ecosystems. Read more stories like this at hakaimagazine.com.
The northeast Pacific Ocean is home to an astonishing array of marine creatures—spiky urchins, multiarmed sea stars, soft sea slugs the color of lemons, and barnacles with their heads glued to rocks. Strolling the seashore or diving below the ocean’s surface, we can see the adult forms of these creatures, but what about their earlier stages? Before they settled down—literally—and moved to the seafloor, almost all started life as zooplankton, marine fauna adrift on the ocean’s currents.
Although zooplankton and phytoplankton, the plantlike drifters that most zooplankton will feast on, are in the water year-round, their populations grow exponentially early in the year. In spring, the days lengthen, the water warms slightly, nutrients flow into the ocean’s surface layers from land or deeper waters, and the phytoplankton bloom. Phytoplankton are microscopic, but as their numbers increase, their chlorophyll and other photosynthetic pigments cause changes in ocean color, which can be measured by satellite imagery and, at high enough concentrations, even become visible to the human eye.
 A satellite image of Vancouver Island, British Columbia, from July 2023 shows colorful plumes of what is likely plankton and sediment. Photo courtesy of NASA
A satellite image of Vancouver Island, British Columbia, from July 2023 shows colorful plumes of what is likely plankton and sediment. Photo courtesy of NASA
The rapid increase of phytoplankton marks the biological spring in the ocean—zooplankton proliferate with the sudden flush of food. Reproductive strategies among animals that produce planktonic babies vary. Many sessile or slow-moving animals, such as corals and sea urchins, use a sort of mass-mailing technique called broadcast spawning. They release billions of gametes in synchrony, with the strategy that at least some of the eggs and sperm will meet up so fertilization can occur. Barnacles, such as the common intertidal acorn barnacle, are hermaphrodites and use a long flexible penis to fertilize the eggs of their neighbor. A fertilized egg develops inside its parent’s protective, volcanolike shell until it hatches into a larva and is ejected out of the house. Whether released as gametes, fertilized eggs, or larvae, they all become part of a seawater soup of microscopic life. While many animals spend only a brief time in the soup before they settle to the seafloor or become more mobile, some species live out the rest of their lives as zooplankton.
Our colleagues at the Hakai Institute’s Quadra Island Ecological Observatory, in British Columbia, spent over three years sampling and photographing zooplankton in the waters off Quadra Island’s eastern shore, a biologically bountiful region where the nutrient-rich outflow of glacial fjords meets the northern extent of the Salish Sea. This research team had a particular interest in capturing larval life forms, which are relatively understudied. It’s astonishing how alien the larval forms of common sea creatures can seem—with their massive, stalked eyes, spiny snouts, and translucent bodies so unlike the adults they’ll become.


Like many marine invertebrates, barnacles have several free-swimming larval stages. They leave the protection of the adult’s shell as a shield-shaped nauplius larva, first photo, with feathery appendages and a single Cyclops eye. The larva will molt six times, adding more segments and appendages. After its final molt, the young barnacle is known as a cyprid larva, second photo. In this stage, the larva sinks to the ocean floor and starts searching for a hard surface on which to attach. Since barnacles need other barnacles nearby for reproduction, the cyprid touches surfaces with its antennae to pick up the scents of others. Once it finds a suitable place, it secretes a dab of glue that secures its head to the surface and grows into an adult barnacle, complete with a conical shell.

This winged beauty is the veliger stage of a predatory sea snail from the family Mangeliidae. The wings, or vela, are covered in microscopic hairs called cilia. The rhythmically beating cilia create currents that entrap food such as diatoms for the growing larva. As the larva grows, it develops the parts more visible in adult snails, such as a shell, foot, and tentacles. Eventually sea snail larvae go through a process called torsion, a 180-degree twist that brings the posterior part of the animal toward its head. As the snail’s planktonic life comes to an end, the veliger settles on the seafloor and develops into an adult.

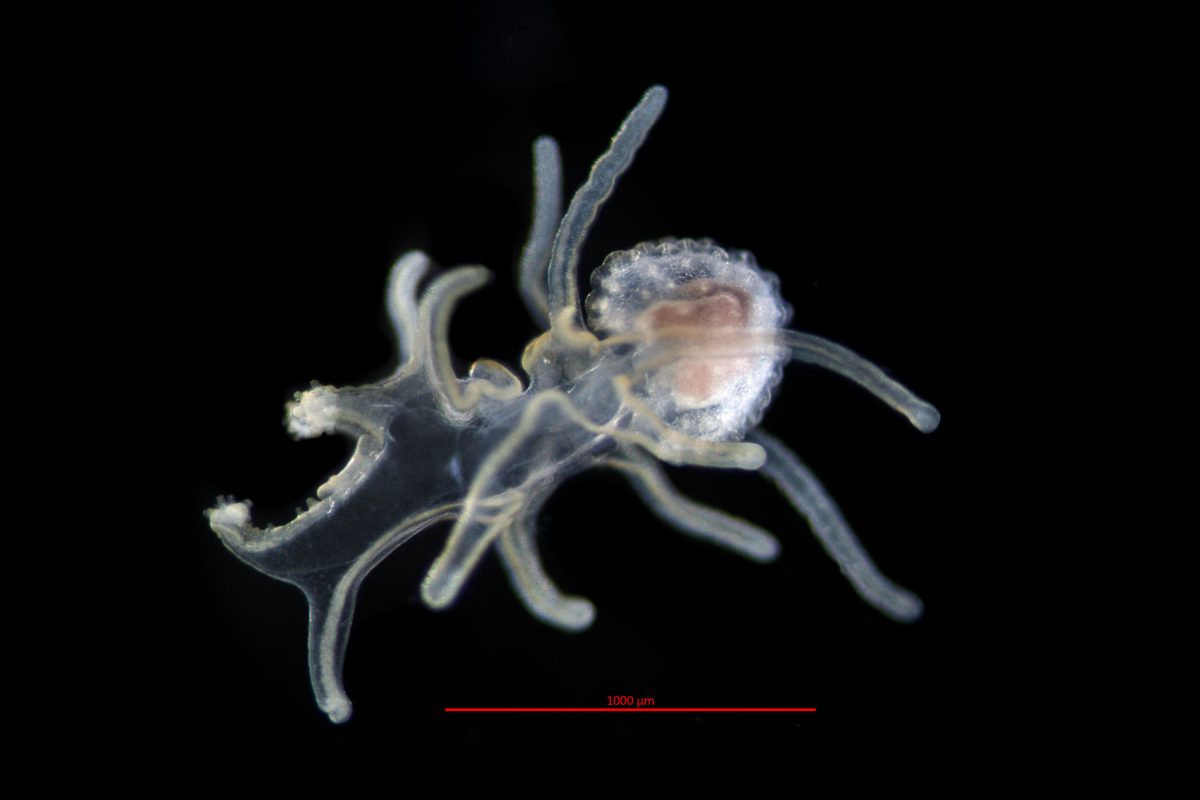
A sea star passes through several stages as a free-swimming larva. At first its body is covered in cilia, which beat in unison to give the larva some control over its motion. As the animal metamorphoses into the bipinnaria stage, first photo, the cilia aggregate into defined bands, and the arms start to form. At this point, its gut also develops, and the larval sea star begins feeding. Depending on the species, a sea star can spend several weeks or months as a bipinnaria before it adds more arms and a sucker at its anterior end and develops into a brachiolaria larva, second photo. The brachiolaria eventually settles to the seafloor, sucker end first, and grows into an adult.
Scientists often categorize animals by their symmetry. Humans, for instance, are bilaterally symmetrical: our right and left halves are mirror images. Up until the point it settles on the bottom, the sea star is also bilaterally symmetrical. As the larva changes into an adult, its symmetry changes, too, morphing into radial symmetry. (Most adult sea stars and their spiny-skinned relatives—the echinoderms—are more specifically pentaradial symmetrical, with a body plan based on five.) New research shows that this shapeshifting is possible because the tissues that are genetically equivalent to a sea star’s “head” are dispersed in its limbs and skin.

While some planktonic creatures are unrecognizable from their eventual adult forms, others come into the world as miniature versions of their parents. This baby opalescent squid is one of hundreds that hatched from a pickle-shaped egg capsule; adult squid lay mounds of these egg capsules in sandy shallows following frenzied mass spawning events, and then leave them to their fate. If the developing jelly-coated eggs survive the gauntlet of opportunistic predators and unforgiving waves and tides, the rice-grain-sized hatchlings, called paralarvae, emerge several weeks later and begin drifting with the currents. Over the following month or so, they gain the swimming skills needed to escape their planktonic lifestyle and hunt in earnest. But in their early days, they can only propel themselves with short pulses of motion as they attack copepods and other zooplankton prey. They’re not fully left to their own devices from the get-go, however—they’re born with an internal yolk sac, a nutritional buffer that gives them time to hone their hunting skills.

This baby moon jellyfish would fit easily on the head of a pin, but it won’t stay this small for long. It belongs to the scyphozoans, or true jellyfish, the typically large and colorful types of jellyfish that most people are familiar with. Far less familiar, however, are their microscopic larval forms known as ephyrae. This snowflake-esque ephyra is one of a stack of clones that budded off its parent polyp—a sessile, stalked stage of the jellyfish life cycle that attaches to rocks, docks, or other surfaces—to drift in the big blue. This process of budding, called strobilation, is perfectly timed with the spring surge in plankton, which means plenty of prey for the new ephyrae. Emerging into the right temperatures seems to be key for their ability to grow. While adult moon jellyfish—which are still considered plankton, despite their size—are found in coastal waters of the northeast Pacific year-round, their ephyrae are a bit pickier about conditions. Too hot and they become stressed and die; too cold and they’ll fail to thrive.

While this might look like a case of jellyfish cannibalism, it’s actually the wild world of jellyfish reproduction in action. Even though it’s no bigger than a fingertip, this adult hydrozoan jellyfish—a relative of true jellyfish—is partway through budding an even smaller mini-me off the wall of its stomach. When adult jellyfish reach maturity, they typically release sperm or eggs, which together develop into polyps; but sometimes environmental conditions, such as temperature, cause hydrozoan jellyfish to change their reproductive tactic and produce mini-mes like this instead. While this style of reproduction isn’t the norm for hydrozoan jellyfish, it’s not rare, either; at least 50 species worldwide are known to sprout clones from one body part or another.


One of the dominant groups among zooplankton, copepods—tiny aquatic crustaceans—are also some of the most abundant multicellular animals on the planet. Most marine copepods are free-living, and many spend their whole lives as plankton, but some species are symbiotic or parasitic on other organisms. These females of the parasitic order Monstrilloida left their bottom-dwelling hosts to reproduce in the water column. Unlike many plankton that take a sink-or-swim approach to reproduction, they offer some parental care to their young. Clocking in at a mere two millimeters long—smaller than a sesame seed—the female in the first photo carries a clutch of bright green eggs that fill most of her body. She can afford to devote this much space to her future babies because the adults don’t feed so they don’t need much of a gut. Instead, they’re physiologically dedicated to creating the next generation of “little monsters” (the etymology of Monstrilla, the first described genus of this group). Once fertilized, the eggs shift outside the mother’s body onto a long trailing spine, as with the female in the second photo, where mucus holds them in place until the baby copepods hatch as nauplii. The nauplii soon leave the plankton community to invade a host and grow up as parasites until they’re ready to continue the cycle.

If this violet tunicate larva looks a bit like a frog tadpole, that’s no coincidence. Tunicates are chordates, animals that at some point in life have a notochord—a precursor to a backbone—as well as a nerve cord. This makes them distant relatives of humans. It’s an unexpected connection, especially because adult tunicates are often colorful, gelatinous blobs. In vertebrates, the notochord and nerve cord eventually become the spine and spinal cord, while in tunicates they disappear after this short-lived larval stage. After incubating for a month inside one of its hermaphroditic parents, the violet tunicate tadpole emerges and stays in the water column for a day or two while it searches for a suitable home to settle onto. Once it attaches head first to a solid surface—whether that’s a pier, piling, or piece of seaweed—the resemblance to its backbone-bearing relations is lost. It resorbs its tail as it transforms into its final form, then quickly gets to work eating and replicating to build a labyrinthine colony.

This “swimming helmet” is the larva of a ribbon worm, an unsegmented marine worm with an impressive retractable proboscis it uses to stab prey. To reproduce, this group of worms—also called nemerteans—respond to chemical cues in the water and gather in mating balls for coordinated spawning. In some species, the fertilized eggs eventually develop into free-swimming larvae, such as this one, called the pilidium. During metamorphosis, a worm-like juvenile develops inside the pilidium. As the worm’s planktonic stage comes to an end, the juvenile breaks out of its pilidium, devours its larval tissues, and heads to the seafloor to begin life as a benthic adult.


To reproduce, a male hermit crab deposits his sperm near a female’s abdomen just after she’s molted. The female will store the sperm until she has produced clusters of several hundred eggs and until conditions are right for fertilization. She then broods the eggs, providing some level of protection. (Females can carry several broods a year.) Young hermit crabs hatch as zoeae, first photo. Long spines and fringed antennae help keep them afloat. A zoea will go through about four molts to become a megalopa, second photo. The juvenile crabs are still tiny at this stage but more closely resemble the adults they’ll become. Since the abdomens of hermit crabs aren’t covered in a hard exoskeleton, the animals slip inside the spiral cavity of an empty snail shell for protection. If food or shells are scarce, hermit crabs will remain planktonic longer before settling to the seafloor.

Like their hermit crab relatives, true crabs start off as spiny-looking zoeae and then metamorphose into megalopae. During this relatively brief final larval stage, crab megalopae are mostly planktonic and use their tail to help themselves swim. But they will also tuck their tail against their abdomen—as shown by this graceful decorator crab—as they make occasional forays to the seafloor to flex their legs and walk in preparation for their fully benthic life. This decorator megalopa will have to wait to test out its namesake skill, though. Adults of the species are among the most prolific decorators in the northeast Pacific. They snip pieces of their environment, like algae and sponges, and adorn their bodies with this camouflaging material using specialized hooked structures. But larvae aren’t able to decorate until they settle to the seafloor for good. As they morph into their juvenile form, they gain their hooks and immediately and thoroughly start to adorn themselves.

The ways in which polychaetes, commonly known as bristle worms, live their lives varies widely, and how they produce the next generation is no exception. The adults of some seafloor-dwelling groups, for example, follow environmental cues, such as the stage of the moon, to partly or fully transform into a swimming version of themselves. For a short time, they become planktonic and gather en masse to spawn in surface waters. Some of these swarming swimmers simply release their gametes near a mate and call it a night. Other species devote more care to reproduction, such as this egg-laden female noble necklace worm that joined a spawning event to gather sperm. Once the eggs she holds in an external brood sac are fertilized, she will swim them back to the seafloor while they incubate. When it’s time for the young to head off into the water on their own, the mother makes a second appearance among the plankton to release her developed larvae.

A hairy worm larva doesn’t exactly sound cute, but baby polychaetes have their charms. This particular grublike creature is a larval parchment tubeworm, a type of polychaete that, as an adult, lives an inconspicuous existence in a long, narrow tube it builds in the seafloor. But first, like other polychaetes, the parchment tubeworm starts out as an active blob called a trochophore. Shaped like spinning tops, trochophores initially have few other distinguishing features besides a head, eyes, and a band of cilia used for movement. As they grow, these larvae start to develop body segments, gain their eponymous bristles, and form more distinguishing shapes—such as this one’s huge mouth and bumblebee-shaped back end.
Acknowledgements
The plankton was collected and photographed by the Hakai Institute’s Biomarathon Project team. Thank you to Tyrel Froese and Matt Lemay from the Hakai Institute; Henry Choong of the Royal BC Museum; and Leslie Harris from the Natural History Museums of Los Angeles County for their expertise and assistance with identification.
This article first appeared in Hakai Magazine and is republished here with permission.
>>> Read full article>>>
Copyright for syndicated content belongs to the linked Source : Popular Science – https://www.popsci.com/environment/pictures-oceans-microscopic-baby-boom/










