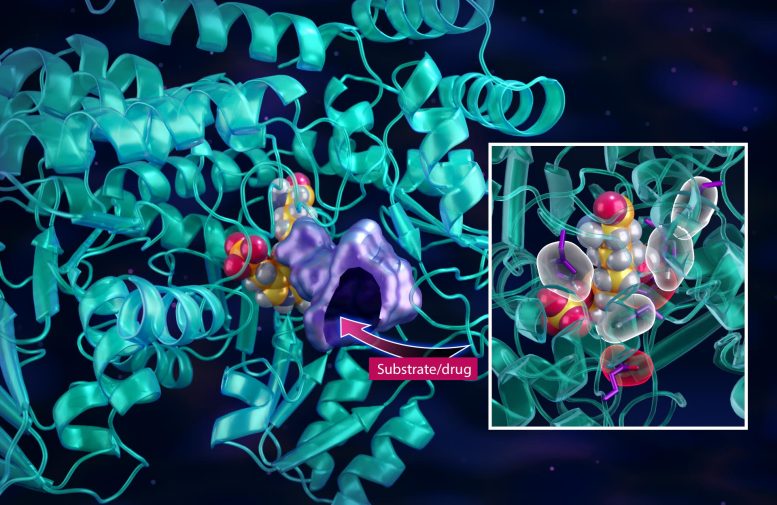
Neutron experiments helped reveal the one-carbon enzymatic mechanism that synthesizes vital food sources for cancer cells that depend on vitamin B6, providing key insights into designing novel drugs to slow the spread of aggressive cancers. Credit: Jill Hemman/ORNL, U.S. Dept. of Energy
Scientists at Oak Ridge National Laboratory are advancing cancer treatment research by designing drugs that target the metabolic pathways cancer cells rely on for growth. By mapping the structure of a key enzyme with neutrons and X-rays, they aim to develop treatments for aggressive cancers, including lung and breast cancer.
After a highly lauded research campaign that successfully redesigned a hepatitis C drug into one of the leading drug treatments for COVID-19, scientists at the Department of Energy’s Oak Ridge National Laboratory are now turning their drug design approach toward cancer.
In a recent study, published in the journal Communications Chemistry, the team used neutrons and X-rays to draw a roadmap of every atom, chemical bond, and electrical charge inside a key enzyme that belongs to a metabolic pathway that cancer cells dramatically overuse to reproduce.
This new information essentially helps pave the way for developing new drugs that act as roadblocks along the metabolic pathway to cut off the supply of vital resources to cancer cells. The drugs would be designed to target highly aggressive tumor-forming cancers that too often become terminal, such as lung, colon, breast, pancreatic, and prostate cancers.
Understanding Cancer at the Atomic Level
“With more than 200 types, cancer continues to be a devastating disease,” said ORNL senior scientist Andrey Kovalevsky. “That means, if we’re ever going to beat the disease, it’s going to require exploring every option and studying every aspect of the disease at every level — from tumors, cells, and molecules down to individual atoms.”
Kovalevsky said this research represents a renewed interest in studying metabolic pathways as targets for developing anti-cancer drug treatments. Metabolic pathways are a series of chemical reactions inside a cell wherein the product of one reaction becomes the base material, or substrate, for the next reaction.
A specific pathway of interest to Kovalevsky and his team is the one-carbon metabolism pathway, or 1C, which uses enzymes that transfer carbon units from one biomolecule to another. This action plays a crucial role in synthesizing important biological molecules such as amino acids, DNA and RNA. In other words, 1C units are like fuel sources that cells need to grow and multiply. That also means they’re vital for the uncontrollable proliferation of cancer cells as well.
“This research is interesting in that the molecules we’re planning to design would be metabolic drugs, which were some of the first drugs — like methotrexate — that were developed to treat cancer. Over the years, research has gone in other directions to study other pathways,” said Kovalevsky. “But recently there’s been a reignition, or return, to the metabolic drugs because you really need a multitude of different intervention options, sometimes at the same time to battle all the different types of cancer.”
Pioneering Drug Design With Neutron Scattering
One of the crucial enzymes within the 1C pathway is serine hydroxymethyltransferase, or SHMT. SHMT is responsible for initiating the lion’s share of 1C reactions for the cell. And, currently, no approved anti-cancer drugs exist that target SHMT specifically.
“The 1C metabolism pathway is ‘hijacked’ by many types of cancer. If you think of this pathway as a highway, SHMT is the on-ramp cancer takes to hijack traffic,” said postdoctoral researcher Victoria Drago, the study’s lead author. “Blocking the enzyme with inhibitors or ‘roadblocks’ prevents cancer cells from using the highway, effectively cutting off their fuel supply, thereby preventing them from spreading.”
But designing a drug requires a detailed understanding of the enzyme structure and how the structure underpins its function at the atomic level. For this, the team used a combination of neutron and x-ray scattering experiments to map the location of every atom in the enzyme structure as well as the network of chemical bonds and the corresponding electrical charges.
Knowing how small molecules attach to the enzyme is the key to designing matching drug molecules — like putting together puzzle pieces in 3D — but the pieces not only have to match in shape but also in electrical charge. Kovalevsky likened it to using the right battery with the correct size and orientation to power specific electronic devices.
In contrast to x-rays, which are more sensitive to heavy elements such as carbon, neutrons are ideal for studying light elements such as hydrogen and are useful in determining the electrical charges and mapping the enzyme-drug interactions.
Neutrons are especially important in that hydrogen atoms make up approximately 50% of all atoms in biological systems, and their presence also plays a significant role in determining the strength of chemical bonds between a drug molecule and an enzyme.
To track the hydrogen atoms, the researchers used the neutron instruments MANDI and IMAGINE at ORNL’s Spallation Neutron Source, or SNS, and High Flux Isotope Reactor, or HFIR. The neutron experiments allowed the team to observe how the SHMT enzyme binds its physiological molecule — serine amino acid — to initiate the chemical reaction, as well as how the enzyme directs the transfer of atoms in the critical steps leading up to the complex reaction sequence. More importantly, the study confirmed how it’s possible to trap serine right before it moves into the pocket where the chemical reaction takes place.
“There have been proposals over the years about the enzyme’s catalytic mechanism and how it functions, but now we know for sure,” said Kovalevsky. “It’s only by pinpointing all the atoms in the active site along the reaction pathway of this enzyme that we gain the knowledge we need to design better drugs that add to the multiple intervention strategies for fighting cancer.”
The research represents a significant first step on the way to realizing a novel drug treatment. The next steps in the research campaign involve studying the enzyme in different reaction stages and testing it against existing drug inhibitors.
The neutron research is part of a larger effort funded by the National Institutes of Health to study a broad class of enzymes similar to SHMT that rely on a single derivative of vitamin B6 to perform more than 140 different chemical reactions.
“The overproduction of SHMT has been linked to the further decline of patients suffering from aggressive forms of cancer,” said Drago. “Developing a more effective treatment that reduces the rate of cancer progression could be just the thing that makes all the difference in someone’s life.”
Reference: “Revealing protonation states and tracking substrate in serine hydroxymethyltransferase with room-temperature X-ray and neutron crystallography” by Victoria N. Drago, Claudia Campos, Mattea Hooper, Aliyah Collins, Oksana Gerlits, Kevin L. Weiss, Matthew P. Blakeley, Robert S. Phillips and Andrey Kovalevsky, 3 August 2023, Communications Chemistry.
DOI: 10.1038/s42004-023-00964-9
In addition to Kovalevsky and Drago, the study’s coauthors include Claudia Campos, Mattea Hooper, Aliyah Collins, Oksana Gerlits, Kevin L. Weiss, Matthew P. Blakeley and Robert S. Phillips. Complementary neutron and x-ray measurements were performed at the Institut Laue-Langevin, France, and the Advanced Photon Source, or APS, at Argonne National Laboratory.
HFIR, SNS, and APS are DOE Office of Science user facilities.
>>> Read full article>>>
Copyright for syndicated content belongs to the linked Source : SciTechDaily – https://scitechdaily.com/this-lab-turned-their-covid-success-into-a-cancer-fighting-mission-heres-how/










