
A UC San Diego study reports a surge in microdosing interest, aligning with policy shifts in psychedelic and cannabis regulation. While public fascination grows, the study calls for comprehensive research to evaluate the health implications and legal concerns surrounding microdosed substances.
New research highlights a significant increase in interest in microdosing, with a 1250% rise in related searches over eight years, correlating with major policy changes in cannabis and psychedelic use.
The study underscores ongoing public intrigue despite limited evidence of benefits, stressing the importance of further research to ascertain the safety and effectiveness of microdosed substances.
Surge in Microdosing Interest Linked to Policy Changes
Americans’ interest in microdosing has skyrocketed with the loosening of local, state, and federal regulations on cannabis and psychedelics. This is according to a new study from researchers at the University of California San Diego.
Published today (June 28) in JAMA Health Forum, the study found that the rate of microdosing-related Google searches grew by 1250% from 2015 to 2023, with over three million searches in 2023 alone. This surge in interest correlates with recent legislative changes decriminalizing or authorizing the use of psychedelic substances in therapy and permitting recreational cannabis use. The research fills a gap in understanding how policy changes affect substance use patterns.
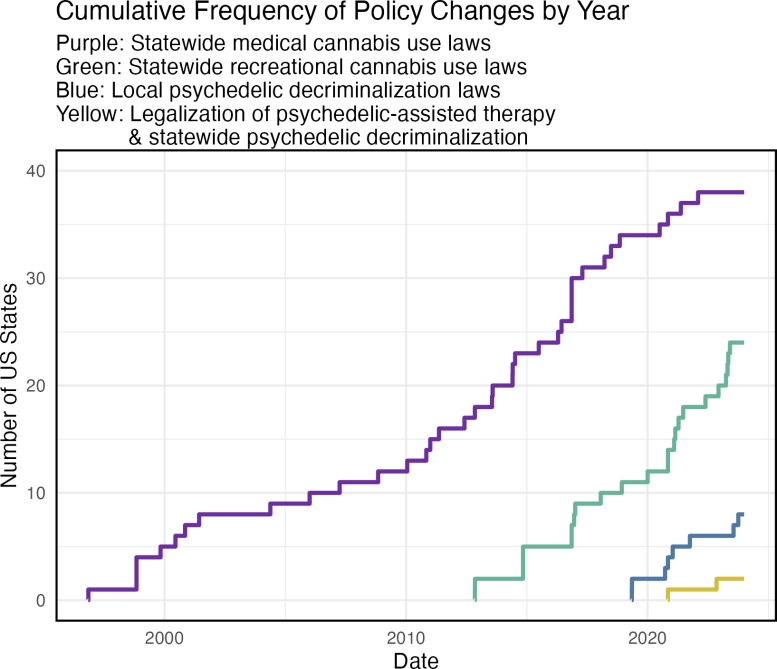
Cumulative frequency of policy changes by US state. Credit: UC San Diego Health Sciences
Microdosing: Perceived Benefits vs. Clinical Evidence
Microdosing involves taking “sub-perceptual” doses of psychedelics, often over prolonged periods, with users claiming it improves cognition, mood, and overall health without causing the intense hallucinogenic effects of higher doses. Despite the lack of clinical evidence supporting these health claims, interest in the practice appears to have grown over the past decade. Current survey data on microdosing are inadequate, prompting the study’s authors to analyze Google search behaviors as a proxy for public interest and to understand how policy reforms have influenced this interest.
The study period coincided with significant policy reforms on substance use. In 2012, Colorado became the first U.S. state to permit recreational cannabis use, and by 2023, 24 states had followed suit, encompassing half of the U.S. adult population. Additionally, eight states had cities or counties decriminalize psychedelic use, and two states legalized psychedelic-assisted therapy and decriminalized psychedelics statewide.
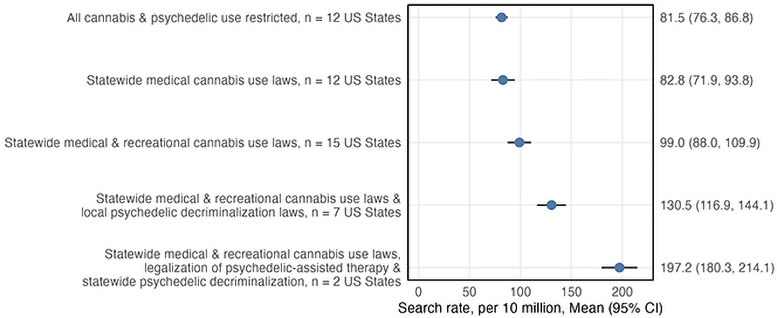
Associations Between Cannabis and Psychedelic Policies and Average Monthly Microdosing Search Rates from August-December 2023. Credit: UC San Diego Health Sciences
Analyzing the Effects of Legislative Changes on Microdosing
Using a dynamic event-time difference-in-difference model, the researchers assessed the causal effects of these policy changes. They used the year before a policy enactment as the reference and states that never adopted the policy as controls. Separate analyses for cannabis and psychedelic policies measured microdosing searches per 10 million Google queries, examining annual and monthly changes in search rates across the U.S.
The results showed that policies reducing criminal penalties for psychedelic and cannabis use were associated with increased interest in microdosing, with the largest increases occurring in states with the most permissive policies, such as Oregon and Colorado. By 2023, these policy reforms accounted for over a quarter of the differences in monthly microdosing search rates across states.
Changing Trends in Microdosing Searches
The study also examined trends in terms related to microdosing, such as substances commonly used for microdosing. Between 2015 and 2018, LSD was the top term related to the microdosing, while from 2019 to 2023, mushrooms were most frequently searched. Other terms included Adderall, cannabis, CBD, DMT, ketamine, and MDMA.
The researchers believe these findings reflect a growing societal interest in psychedelics and psychotropic substances as alternative therapies, possibly replacing evidence-based care.
On a federal level, President Biden suggested reclassifying marijuana as a less dangerous drug in 2022, and the Justice Department approved this recommendation in May 2024. Kevin Yang, M.D., lead author of the study and a psychiatry resident at UC San Diego School of Medicine, believes these actions, along with state and local legislation, indicate a more open attitude toward psychotropics, potentially encouraging scientific investigation.
Call for Rigorous Research on Microdosing
Yang emphasized the importance of rigorous research, stating, “As public interest in using psychedelics and cannabis for health grows, it’s crucial that the medical community conducts studies to establish a strong evidence base for their safety and efficacy. Without understanding the risks and benefits, people may turn to unproven alternative therapies, exposing themselves to potential dangers. It’s our responsibility as a medical community to ensure patients have access to safe, effective, and evidence-based treatments.”
Legal and Health Concerns Over Microdosed Products
The authors expressed concern about the market for microdosed products. “Psilocybin and nearly all commonly microdosed substances are Schedule 1 controlled substances. Using these substances poses legal risks for consumers and concerns of product impurity because of a lack of manufacturing standards,” said Eric Leas, Ph.D., M.P.H., assistant professor in the UC San Diego Herbert Wertheim School of Public Health and Human Longevity Science and senior author of the paper.
He noted that products claiming to be “magic mushrooms” for microdosing might not disclose ingredients or could contain harmful substances like Amanita muscaria, which lacks clinical support as a therapy and can be toxic.
Future Research and Consumer Education Needed
The researchers have conducted a nationally representative cross-sectional survey of three commonly microdosed substances for a similar analysis and are calling for more clinical studies on microdosing’s effectiveness. They emphasize the need for safe products, well-informed consumers, appropriate manufacturing practices, and documented benefits and risks.
“We need to better translate clinical evidence for consumers and policymakers to understand the benefits and risks of microdosing and how policy changes drive interest in substance use,” said Leas.
Reference: 28 June 2024, JAMA Health Forum.
DOI: 10.1001/jamahealthforum.2024.1653
Co-authors include: Nora Satybaldiyeva, Matthew R. Allen, John W. Ayers, of UC San Diego.
Funding/Support: The work is supported in part by grant T32IP4684 from the California Tobacco Related Disease Research Program, grant K01DA054303 from the US National Institute on Drug Abuse and Burroughs Welcome Fund.
>>> Read full article>>>
Copyright for syndicated content belongs to the linked Source : SciTechDaily – https://scitechdaily.com/tiny-doses-massive-interest-1250-surge-in-microdosing-as-drug-laws-evolve/










