When a woman goes through menopause—defined as not having a period for at least 12 months—the health impacts are immediate and dramatic.
That’s because ovaries (aside from their reproductive function) are endocrine organs. And when they stop pumping out the cocktail of chemicals that communicate with almost every tissue in the body, everything from the brain to the muscles to the skin is affected.
“Your risk of osteoporosis goes up overnight, your risk of cardiovascular disease goes up overnight,” says Jennifer Garrison, a neuroscientist at the Buck Institute for Research on Aging in California. And this seismic shift often hits between the ages of 45 and 55 (average age of onset is 51), coinciding with a woman’s peak years in the workforce. A study released by the Mayo Clinic estimates that in the United States, menopause is responsible for up to $1.8 billion in lost work time and more than $26 billion in medical costs.
“Ovaries are the architects of healthy aging in women,” Garrison says. So, it doesn’t make sense to talk about women’s health and longevity without considering reproductive longevity. For example, why do ovaries, which begin to show signs of age in a woman’s 30s, deteriorate decades earlier than other organs? Why do some people reach menopause earlier or later than average? And most importantly, if we could delay menopause, by keeping the ovaries functioning for longer, would that translate to more years of good health?
Unfortunately, for a long time research on reproductive health was laser-focused on fertility and the child-bearing years. David Pepin, a reproductive biologist at Harvard, recalls meeting with the U.S. Food and Drug Administration five years ago to discuss funding.
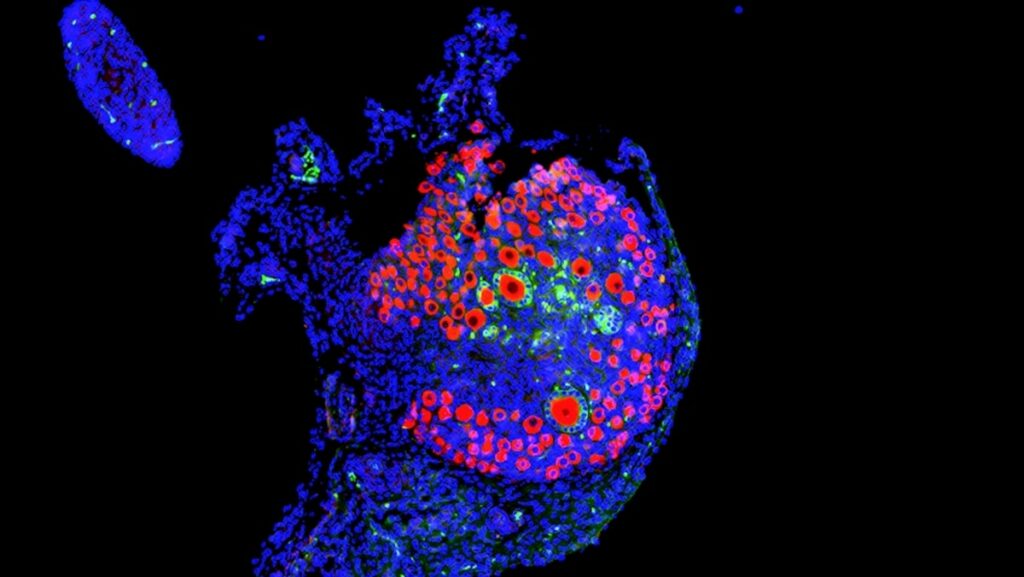
A normal mouse ovary shows many immature eggs—or oocytes—in red. Larger oocytes surrounded by green hormone-producing granulosa cells indicate growing follicles; one oocyte will usually grow large enough to be released during ovulation.
Micrograph by David Pepin
“I couldn’t convince them that having your ovaries function and make hormones is—in itself— important; that the ovaries are not just to make babies,” says Pepin. As a result, he’s had to be resourceful. Some of the work he’s done on the life cycle of ovaries has been funded by its applications for controlling the population of domestic cats.
But the tide is turning.
“There’s been an exponential increase in interest and research” in the last five years, says Garrison, who in 2020 co-founded the Global Consortium for Reproductive Longevity and Equality, an initiative that funds scientists, fosters collaborations, and educates the public about the crucial role of reproductive health in women’s health. “Suddenly, at aging research conferences, reproductive aging is represented now, whereas it wasn’t two or three years ago.”
Why ovaries matter
Healthy ovaries produce a series of molecules that send signals to distant organs to help them function. Among the best studied is estrogen, which ebbs and flows with the menstrual cycle and peaks in the days before ovulation. But estrogen receptors—the molecules that grab estrogen, triggering downstream actions in cells and tissues—are present throughout the body, extending estrogen’s reach far beyond the reproductive organs.
When estrogen binds to its receptor, the resulting complex acts on DNA to switch some genes on, and others off. Estrogen, therefore, has its fingers in a lot of pies. In the cardiovascular system, the hormone helps widen blood vessels and ensure their lining is smooth and slippery, which lowers blood pressure and prevents clots from forming. In the brain, it’s neuroprotective—dampening inflammation, promoting healthy synapses, and clearing away misfolded proteins. In the musculoskeletal system, estrogen helps build and repair muscles as well as maintain bone.
(What happens during menopause? Science is finally piecing it together.)
The loss of estrogen, therefore, puts women at increased risk for developing diabetes, cardiovascular disease, dementia, osteoporosis, and more. On the flip side, individuals who become menopausal later in life compared to their peers tend to live longer and healthier. This benefit extends even to their male siblings, pointing to a potential genetic link between reproductive health and overall longevity.
How ovaries age
Although scientists have characterized some consequences of ovarian failure—both premature and as a part of normal aging—the processes that drive it remain mysterious.
What we do know: At puberty, when the ovaries contain approximately 400,000 follicles, the brain begins to communicate with these organs and activates up to a thousand dormant follicles—fluid-filled sacs that house a developing egg—each month. Of these, a handful mature, producing hormones like estrogen and progesterone, which send signals to the brain to prepare the uterus for a possible pregnancy.
Most of the growing follicles wither and die; but each month one (and sometimes two or three) will fully mature and release an egg for potential fertilization. This process repeats every month until menopause, when fewer than a thousand follicles remain.
(Scientists are finally studying women’s bodies. This is what we’re learning.)
Years before menopause, however, the feedback mechanisms between the brain and the ovaries (and the follicles they contain) become chaotic as follicle numbers dwindle, Pepin explains. “But it’s a complete black box” as far as how this impacts the trajectory of ovarian aging or whether it differs in different people, he says.
If these feedback mechanisms are important, though, one way to preserve healthy ovarian function may be to hold onto the follicles you have.
Pepin demonstrated that anti-Müllerian hormone, which is made in the follicles and controls the number that are activated (and therefore eventually lost in a menstrual cycle), can do just that.
When mice exposed to chemotherapy (which seems to jumpstart the development of dormant follicles, increasing the pool of eggs that die in each cycle) were injected with the hormone, fewer follicles were activated and more eggs were kept in reserve than controls that received saline.
Similarly, when a short two-week course of rapamycin—a drug that also prevents dormant follicles from developing—was given to female mice, the compound was shown to extend fertility, particularly in older mice (equivalent to humans in their late 40s), and increase the number of follicles in reserve.
Another benefit of rapamycin use: egg quality improved. In other words, eggs from treated mice had fewer chromosomal abnormalities and healthier mitochondria.
Yousin Suh, a geneticist at Columbia University, believes rapamycin holds a lot of promise. Used to treat some cancers, rapamycin has a strong safety record—smoothing the way for testing it in other contexts.
Suh and collaborators are currently conducting a Phase II clinical trial that will measure the ovarian reserve of subjects—between the ages of 38 and 45, when about 20,000 follicles remain—after three months of treatment with the drug.
But rapamycin’s power may extend beyond its ability to suppress follicle activation. The molecule also targets, or blocks, the processes (controlled by a molecule called mTOR) that regulates cell growth and metabolism across species as varied as flies, mice, and humans. When those mechanisms are overly active, they prompt cells to divide and proliferate, which is why these mTOR-linked activities are implicated in aging and cancer.
That’s why it makes sense that blocking them could translate to greater longevity. And in studies on middle-aged mice, rapamycin did decrease inflammation and increase lifespan. Rapamycin’s effect on ovarian aging, therefore, could be two-fold, says Suh.
The environment of the egg
As a geneticist, Suh is relatively new to reproductive biology. She switched fields when she was recruited to Columbia in October 2019.
“I had no biases; I knew nothing except for the fact that I was reproductively aging, and it was horrendously bad,” Suh says. She approached the problem from her unique lens, seeking to identify the genes and molecules that were most and least active in the aging ovary.
“What we found is that in the entire tissue of the ovary, across all cell types, you see the screaming signature of mTOR activation,” Suh says.
And it’s not just mTOR. Pathways for cell communication, mitochondrial function, and DNA repair, were markedly different between young and old ovaries. Clearly, follicles and eggs are only part of the story; the environment they’re sitting in may be just as important.
Francesca Duncan, a reproduction biologist at Northwestern, happened upon this insight almost by accident. She started her career as an egg biologist, working on eggs isolated from mice and throwing out the rest of the ovarian tissue. But egg-containing follicles are nestled in an environment of specialized cells that nurture and support them. Considering one without the other paints an incomplete picture.
“It wasn’t until we started to compare eggs from young and old mice that we realized, it’s just hard to get follicles out of an old ovary compared to a young ovary,” Duncan says. In fact, how difficult it was to pop a follicle out of its matrix was a pretty reliable indicator of how old the animal was. This epiphany changed the trajectory of her work and revealed a new avenue of research.
With age, Duncan and others discovered, the ovarian environment becomes stiff and fibrotic—a process that also occurs in other tissues that fail over time like the liver, lungs, and heart. Stiffer ovaries impede follicles from growing, which affects fertility, decreases the production of hormones that keep women healthy, and torpedoes egg quality.
Think of it like eggs in a nest, Duncan says. “If you can create a hospitable, suitable nest, that would sustain egg function and endocrine function much longer.” The idea that a cell’s environment could influence its behavior is not without precedent. The tumor environment, for example, often dictates how aggressive cancer cells become.
Underscoring this point, a group in Australia showed that acute treatment with anti-fibrotic drugs used for pulmonary fibrosis can reverse ovarian aging and restore ovulation in older mice. Duncan’s team achieved similar results with a longer-term, lower-dose course intended to extend ovarian lifespan.
Future treatments
Although there’s so much more to learn, scientists are hopeful that progress is real and accelerating. “It’s wild to me” that less than a decade after our first observational paper on ovarian stiffness in 2016, we’re thinking about clinical trials for anti-fibrotic therapeutics and biomarkers, says Duncan.
She’s most excited that the solutions being discussed go beyond the current standard practices: egg and embryo freezing (for fertility) and hormone replacement therapy (for endocrine function), which just chip away at symptoms but don’t address the root cause.
“I’m not personally interested in a band-aid solution; I want the ovary to maintain its normal function longer,” Duncan says.
The fate of ovaries is more complex and their impact on well-being more nuanced than ever imagined. Even after menopause, for example, ovaries are doing something: Menopausal women who undergo oophorectomies are at greater risk of coronary disease and death compared to peers with their ovaries intact.
According to Garrison, it’s crucial to investigate what happens to the ovary across the entire adult lifespan—from puberty to the fertile years to menopause and beyond. In an ideal future, she says, “we would have interventions and treatments and therapies available for every single one of those life stages.”
>>> Read full article>>>
Copyright for syndicated content belongs to the linked Source : National Geographic – https://www.nationalgeographic.com/premium/article/slow-aging-ovaries-delay-menopause-boost-brain-heart-health