1. Historical perspective
In edible oil processing, a fractionation process consists of a controlled cooling of the oil, thereby inducing a partial, or ‘fractional’, crystallization. The remaining liquid (olein) is then separated from the solid fraction (stearin) by means of a filtration or centrifugation.
This kind of fractionation process has been applied for almost 150 years. In most literature, Hippolyte Mège-Mouriès is credited with the invention of “a patented method to produce certain fats of animal origin”. In fact, he concocted the production of a sort of margarine fat, by separating a liquid fraction from ordinary tallow after gentle cooling. But with only temperature difference as the driving force, a fractional crystallization of a fat is a perfectly natural, spontaneous phenomenon. So it was also observed that in palm (kernel) oil harvested in tropical regions, small crystals would appear upon cooling and form a crystal suspension in the wooden barrels during shipping to chillier Western Europe. These slightly denser solids eventually settled, and such fractions could effectively replace hardened fats in margarines [1]. In a more evocative twist, we could therefore consider these wooden shipping drums the very first oil crystallizers, with just the peaceful ocean waves providing the necessary agitation to keep the mix in suspension. Moreover, the natural fractional crystallization of fats when mildly cooled is echoed in the term ‘winterization’, referring to the habit of leaving large oil tanks quiescent in wintertime to induce some mild crystallization and obtain a liquid fraction with improved cold stability, in an economic fashion [2].
Despite the apparent spontaneity of the process itself, it took until the 1960s for the fractionation industry (and technology) to boom, when the production of palm oil in Southeast Asia heavily increased and export taxes on processed palm oil were reduced. At that time, however, the boundaries of the technology were mainly determined by the phase separation. In the early stages of fractionation technology, the olein and stearin fractions of oils and fats had to be separated by settling, using only the force of gravity to bring about a separation between the heavier solid phase and the lighter liquid phase, which left the settled solid phase containing large quantities of entrained (trapped) liquid oil, almost certainly more than 75% [3]. In the last decades, the continuous development of separation techniques, from vacuum belt filtration to centrifuges and membrane press filters, has put fractionation on the map as a versatile and economic modification technique. Although some specific techniques relying on use of detergents are still applied for very particular production, actually only two main fractionation technologies are used in the 21st century’s edible oil industry:
Dry fractionation, also known as crystallization from the melt, is fractional crystallization in its most simple form, and the economy of the technology allows it to be used for production of commodity fats. Dry fractionation has long been regarded as an unpredictable, tedious and labor-intensive process. However, the relatively cheap dry fractionation technique has evolved to the modification technology of the 21st century [4], as without additives, polluting effluents or post-refining involved, the sustainability and safety of the process aresecond to none.
Solvent fractionation, already patented in the 1950s, involves the use of hexane or acetone to let the high-melting components crystallize in a very low-viscosity organic solvent. This can be helpful with respect to the selectivity of the reaction, but mainly offers advantages in the field of phase separation: Much purer solid fractions can be obtained, even with a vacuum filtration. Being a more expensive process, it is less common than dry fractionation and only comes into the picture when a very high added value of (at least one of) the resulting fractions makes up for the high cost.
In this contribution, the emphasis is on dry fractionation technology.
2. The Crystallization Stage
Principal concepts
A controlled crystallization of the melt is the backbone of any dry fractionation process, and the success of this process relies principally on the phase behavior of the constituent triglyceridess. Therefore, it is probably useful to dedicate some words to that part of the physical chemistry of fats and oils that lies at the basis of the fractionation technology.
If anything is clear from browsing through the Lipid Library, it is the fact that a natural oil is a very complex mixture of different triglycerides (not to mention diglycerides, free fatty acids, phospholipids, sterols, and uncountable other minor constituents). The mix of triglycerides does have a repercussion on the melting behavior of a fat: It does not exhibit a sharp melting point, but often displays a steady softening (or increasing liquid content) with increasing temperature, until it is completely liquid. The position and width of this melting range on a temperature scale is determined by the type of constituting triglycerides and compositional heterogeneity of the oil:
The type of the triglycerides: in a very simple approach, the longer the fatty acid moieties, the larger the total molecule and consequently, the more energy (i.e. higher temperature) will be required to convert such triglycerides from a solid to a liquid ‘state’. A double bond in the carbon chain decreases the melting point dramatically, however, and this is why oils containing a high proportion of unsaturated fatty acids are generally liquid.
The broader the spectrum of triglycerides present in the oil, the broader the melting range. Some of the triglycerides will only solidify (or melt) at 5°C, whereas others will still be hard as candle wax at room temperature.
Another matter to take into consideration is the respective concentration of each of these triglycerides. This makes two variables to consider if a certain triglyceride will remain in the melt or crystallize: the temperature and its concentration (in fact, just the same principle applies for sugar in coffee). So, ideal solubility calculations based on the melting temperature and enthalpy of the pure solute, as well as the absolute temperature as the principal variables, can serve as a first approach to the solubility of a triglyceride in a solvent. But this premise of ideality is the real issue in fractional crystallization of oil: These are not regular solutions, the triglycerides are not dissolved in an ‘inert’ solvent; they are dissolved in a melt, i.e. other triglycerides. Thus, the fact that the solvent and solute have quite some structural similarity leads to considerable deviations from the ideal solubility. The most relevant is the occurrence of “intersolubility” in a solid state: the property to form a solid ‘solution’ in which the constituting triglycerides cannot be separately determined, nor divided: It behaves as one phase. Consequently, such intersolubility of the triglycerides often presents the largest fundamental problem in several fractionation processes, as the actual goal of fractionation is to separate different triglycerides selectively.
It is fair to state that other typical fat crystallization phenomena such as polymorphism are of secondary importance compared to intersolubility; typical fractionation conditions are generally sufficiently restricted in time and temperature range to only allow one type of molecular arrangement to form. For palm oil, this is typically in a β’-form from start to finish.
Intersolubility is also increased at higher degrees of supercooling, so if the fractional crystallization is to occur selectively, the challenge for the process engineer is to steer clear from such conditions and keep the melt just deep enough in metastable conditions to create a driving force for crystallization of the triglycerides of interest, but not too deep as to prevent formation of solid solutions or uncontrolled crystal growth. If the crystal growth is well controlled, the crystal aggregates result in sharply discrete and dense spherulitic structures, sometimes measuring up to several millimeters in diameter, which are fairly uniform in size and shape (Fig. 1).
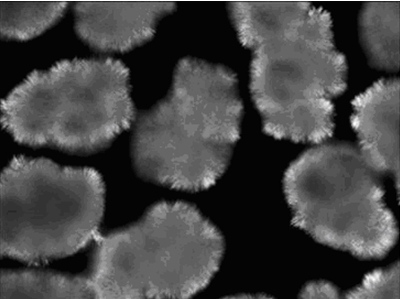
Figure 1. Polarized light microscopic picture of typical spherulitic crystals developing in palm oil fractions.
To be complete, the influence of minor components is not one to be captured in one phrase, but overall ‘impurities’ such as diglycerides have a negative effect on crystal growth and filterability of the formed solids. Also product-wise, it should be kept in mind that dry fractionation is a ‘rubbish in, rubbish out’ process, so a feedstock with a lot of impurities will never result in two clean fractions.
Conducting fractional crystallization
The basic principles sketched in the former paragraphs help to explain the key aspects of a crystallizer, the technological heart of the fractionation installation. It should be able to gently cool down a mass of oil (up to 100 ton/batch) and keep the resulting crystal suspension as homogeneous as possible. Note that such gentle cooling means in fact imposing very low supercooling conditions, and it will result in a formation of fewer and larger crystals, because the said conditions simply rule out the existence of a mass of tiny crystals. Fat crystallization is a fairly exothermic reaction (up to 180 kJ can be released for every kg of crystals formed), so the efficiency with which this energy can be removed is an important design feature. For most industrial crystallizers, this ranges between 120 and 200 W/m2·K.
The problem with occurrence of excessive intersolubility at higher degrees of supercooling explains the very need for a (in terms of temperature) homogenizing crystallizer. Too steep temperature gradients within the crystallizer will lead to formation of viscous solid solutions near the cooling wall (the coldest spot), while oil entrapped in dead zones or with insufficient heat exchange with a cooling wall could encounter too high temperatures to crystallize at an acceptable rate. Note that two extreme temperatures will not result in an average degree of crystallization, but in a viscous slurry of badly filterable solid solution-crystals in a matrix of unstable, hazy liquid. At these unfortunate occasions, it is comforting to remember that a dry fractionation process is 100% reversible, and that upon heating, this mess will revert to the melt again, ready for another attempt….
In order to preserve the selectivity and to avoid the temperature gradients within the melt, the cooling rate under crystallization conditions is quite slow (0.2-3°C/h), depending on the sensitivity of the reaction and the performance of the crystallizer. Sensitivity of the reaction might be a little of a subjective term, but it can be quite elegantly illustrated by Figure 2. This shows a differential scanning calorimetry profile of palm oil (full line above) and palm olein (dotted line below).
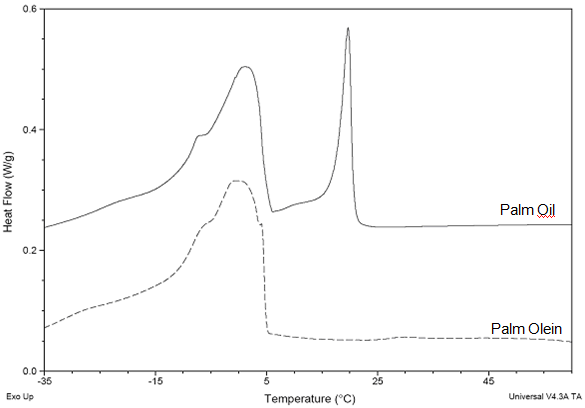
Figure 2. DSC cooling profile (-5°C/min) of palm oil and its derived palm olein fraction. Exothermal peaks are shown upwards.
The upward peaks in the profile show at which temperatures and how much heat of crystallization will be released upon cooling (here at 5°C/min!). For palm oil, the sharp peak on the right is created by the fast solidification of predominantly trisaturated triglycerides. It is largely (and not solely, as some intersolubility will inevitably occur) this fraction that is crystallized in the first step or ‘cut’ in multistage palm oil fractionation. Although the absolute temperatures in this diagram are not really relevant for fractionation because of the high cooling rate, the diagram suggests clearly how simple fractional crystallization can be: You put the temperature in between the two peaks and wait for the solidification to take place. When this first trisaturated fraction is removed, however, the remaining palm olein shows one large peak, and it is instantly clear that a slow fractional crystallization of a distinct fraction is a lot harder to accomplish in this matrix: There is a lot more material to crystallize in a narrow temperature region, and intersolubility will have its say.
The cooling medium removing this heat of crystallization from crystallizers is typically clean cooling tower water, sometimes mixed with some propylene glycol to be able to work at subzero conditions (as in fish oil fractionation). Cooling by ammonia evaporation can also be considered, but very often turns out to be too expensive for a classic installation. The cooling wall itself can be double-jacket, stainless steel cooling fins (plates) or pipes. Normally, a cooling surface of at least 4 m2 per m3 oil is expected to ensure proper heat transfer for bulk edible oil fractionation.
In programming the cooling regime, generally three ‘temperature’ parameters can be kept in check: the cooling medium temperature, the oil temperature or sometimes also ΔT, the temperature difference between oil and cooling medium. The subtlety of the process then exists in knowing which cooling rate and cooling modus to apply at which exact stage in the process, for there is no art in crash-cooling the lot. This is where the process technologist can make a true impact on the outcome of the process, as a difference in cooling regime could lead to a different process cycle time, filterability of the slurry and overall economy of the process.
For a given cooling rate and heat exchange surface, the appropriate removal of released crystallization heat to the cooling medium is also function of the agitation and the viscosity of the bulk. The first being an externally imposed process feature, the type and intensity of agitation is indeed a typical ‘design’ matter and linked with the concept of the crystallizer. It should be sufficient to enhance heat transfer and favor crystal initiation and secondary crystallization, but on the other hand excessive agitation could damage the structures of the crystals being formed, which might present problems in the subsequent filtration stage.
Viscosity, on the other hand, is an intrinsic state of the oil (though to be fully correct, an edible oil crystal suspension does exhibit some shear-thinning behavior). Excessive viscosities will reduce mass and heat transfer between solid and liquid, hence decreasing the crystal growth rate, also by limiting molecular diffusion. The flow properties of the oil not only are a function of solids concentration present in the liquid, but are also greatly influenced by the interactions between the different crystal entities such as network formation and/or ‘gelly layers’ [5]. In normal fractionation conditions, the viscosity of crystal suspensions just prior to filtration ranges from 300 to 2000 mPa·s depending on the application, though in palm kernel fatty acid fractionation, viscosities over 50,000 mPa·s are not exceptional.

Figure 3. A developing oil/crystal suspension. The darker wakes indicate ‘spontaneous separation’ of the liquid from the bulk upon stirring.
3. The Separation Stage
Although the triglyceride separation theoretically is already established during crystallization, it is clear that the separation stage itself effectively determines the product yields as well as the stearin quality. As more residual olein can be expelled from the solids cake, the final stearin will be more concentrated in crystals and will turn out ‘purer’ and will display higher and steeper melting. The olein quality is determined entirely by the amount and selectivity of crystallization in the preceding stage. In some applications, the formed crystals are often not sufficiently stress-resistant and get squeezed through the filter medium. Obviously, such contamination of crystals in the olein phase affects the efficiency of the fractionation process negatively and results in a liquid phase with inferior cold-stable properties. Overall, the ‘permitted’ degree of olein dilution in the stearin cake determines the choice for the applied separation technology, exemplified in Table 1.
Table 1. Different separation systems for palm oil fractionation [6]
Vacuum filtration
Centrifugal nozzles
Membrane press (16 barg)
IV Palm oil
52
52
52
IV Palm olein
56-57
56-57
56-57
IV Palm stearin
40-42
36
30-32
Solids in cake (%)
46
–
65
Olein yield (%)
72
76
82
Membrane press filtration, as also used in for example sludge dewatering systems, is by far the most used separation technology in dry fractionation nowadays. Such filters consist of a large steel frames that can easily hold up to 150 filter plates together, each plate counting for up to 7 m2 of filtration surface and over 100 L filter chamber volume (Fig. 4).

Figure 4. A membrane press filter used in dry fractionation.
Usually, the filter chambers are first filled with the crystal suspension, and in doing so a large portion of the liquid olein is already passing through the filter cloths. Then watertight membranes (one membrane per chamber) attached to the internals of the plates are gradually inflated (with water, liquid oil or air) like internal balloons to the desired pressures, reducing the chamber volume and pushing out residual liquid, which is immediately evacuated via internal channels in the plate towards collecting tanks. The volume reduction of the chamber thus literally compacts and dries the cake. Typical final squeeze pressures are 6 or 15 barg, 30 and even 50 barg membrane press filters are available on the market to attain higher purity in speciality fat cakes. It is also good to note that the mass fraction of solids in the filter cake decays exponentially as a function of the distance to the filter cloth, and consequently thinner filter chambers and longer squeezing times can be helpful (yet costly) means to reduce the entrainment significantly.
The whole filtration plus squeezing operation can vary from 30 to 90 minutes. After this, the filter plate package is opened so the solid cakes can just drop by gravity into a stearin tank underneath the filter to melt.

Figure 5. Hopper and stearin tank, mounted underneath the membrane press filter.
4. The Fractionation Plant Assembly
Figure 6 presents a general layout of a present-day dry fractionation process. Often multiple crystallizers are used in (overlapping) series. This is not only a matter of capacity, it is also in order to maximize the use of the filter; by a good planning of the crystallization times of filtration, the expensive (batch) filter should be in constant operation.
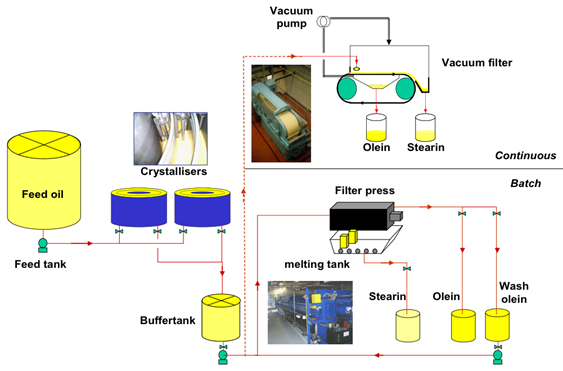
Figure 6. Layout of a typical dry fractionation process.
The reduction of dead time of a filter can also be established by means of a crystallized offer buffer tank; each crystallizer can be quickly drained and made ready to receive the next batch of oil, while the cooled buffer tank will send set volumes of crystal slurry to the filter, whenever it is ready. Continuous filtration systems have been a very elegant strategy in dry fractionation as well, although currently, the demand for purer solid fractions as obtained by filter chamber compaction has pushed continuous belt filters somewhat out of the dry fractionation market.
It should be kept in mind that fractional crystallization of a triglyceride oil is a relatively slow process and is therefore the time-determining stage; some simple fractionations can be established in about 5 hr crystallizer residence time, whereas more complex oils can require up to 3 days of cooling and crystal maturation before being sent to the filter.
5. Applications and Future Developments
In essence, the goal of fractionation is to create the biggest possible difference between two fractions. Palm oil is by far the most fractionated oil in the world. Given the broad spectrum of triglycerides and also its naturally high amount of palmitic acid that gives a fat ‘body’ at room temperature, the separation of palm oil into sharply defined fractions usually happens in a multi-stage process (Fig. 7).
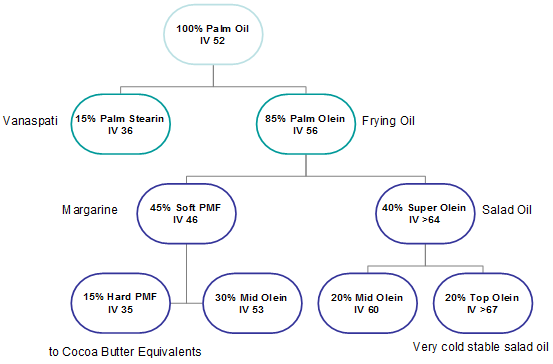
Figure 7. Multistage fractionation of palm oil with possible food applications for the various fractions.
The first step of dry fractionation of palm oil yields olein fractions with a cloud point below 10°C. The olein fractions are used as a substitute for soft oils in frying, cooking and salad oils or are being further fractionated. Together with a further development of single-stage palm oil fractionation by technological improvements, there is an increased tendency to execute a double or triple fractionation of palm oil in order to produce fractions with specific characteristics such as high IV superoleins (IV> 65) and hard palm-mid-fractions (hard PMF) (IV
The latter fraction can serve as a feedstock for the production of typical cocoa butter equivalents (CBE), which are non-lauric fats similar in their physical and chemical properties to cocoa butter. They are often prepared by solvent fractionation [7], though the more contemporary developments within dry fractionation (better suited crystallizers, improved separation technologies) are closing the gap between the quality of solvent- and dry-fractionated hard PMF.
Another technological field of interest is the use of plug flow reactors that allow the fractional crystallization in a continuous fashion, offering considerable reduction in operation costs (such as steam usage and cooling power). Indeed, just as in any other edible oil processing technology, there is a continuous quest for economization and process optimization. Heat recovery systems, crystal seeding installations, optimized mixing procedures and elegant plant layouts can all contribute to maximize capacity and minimize costs for a dry fractionation plant.
One final comment is that the process technologist should always remember that a fractionation process yields two products, and thus that the sum of the value of the two fractions should always exceed the processing cost and feedstock cost. This is why the feasibility of multistage fractionation is not only a matter of technological know-how, but also a matter of having markets for all ‘by-products’ generated along the way.
References
Rossell, J.B. Fractionation of lauric oils. J. Am. Oil Chem. Soc., 62, 385-389 (1985) (DOI: 10.1007/BF02541409).
Illingworth, D. Fractionation of fats. In: Physical Properties of Lipids, pp. 411-477 (A.G. Marangoni and S.S. Narine (eds.). Marcel Dekker, New York, USA) (2002).
Hamm, W. Entrainment, are we making progress? Lecture Paper Series. Society of Chemical Industry (2005).
Timms, R.E. Fractional crystallization – the fat modification process for the 21st century. Eur. J. Lipid Sci. Technol., 107, 48-57 (2005) (DOI: 10.1002/ejlt.200401075).
Calliauw, G., Fredrick, F., Gibon, V., De Greyt, W., Wouters, J., Foubert, I. and Dewettinck, K. On the fractional crystallization of palm olein: Solid solutions and eutectic solidification. Food Res. Int., 43, 972-981 (2010) (DOI: 10.1016/j.foodres.2010.01.002).
Krishnamurthy, R. and Kellens, M. Fractionation and winterization. In: Bailey’s Industrial Oil and Fat Products, Volume 4, Edible Oil and Fat Products: Processing Technology, pp. 301-337 (Y.H. Hui (ed.), John Wiley, Champaign, USA) (1996).
Hashimoto, S., Nezu, T., Arakawa, H., Ito, T. and Maruzeni, S. Preparation of sharp-melting hard palm midfraction and its use as hard butter in chocolate. J. Am. Oil Chem. Soc., 78, 455-460 (2001) (DOI: 10.1007/s11746-001-0285-0).
>>> Read full article>>>
Copyright for syndicated content belongs to the linked Source : Hacker News – https://lipidlibrary.aocs.org/edible-oil-processing/dry-fractionation










