The scientific team at the National Institute of Animal Biotechnology (NIAB), under the guidance of Syed Faisal, is dedicated to creating a cutting-edge vaccine targeting this critical zoonotic disease.
| Photo Credit: The Hindu Graphics | Subyendu Ganguly
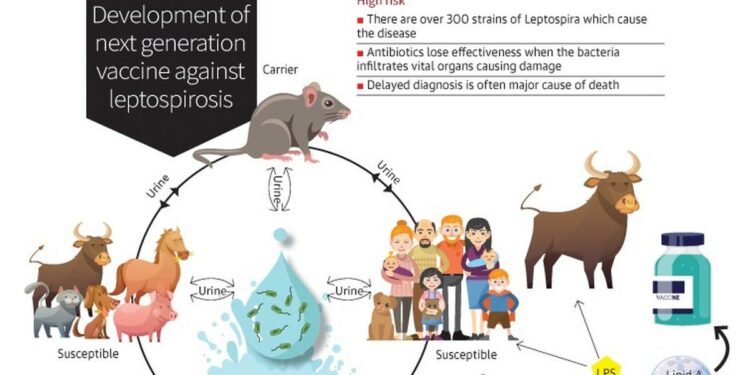
Researchers at the National Institute of Animal Biotechnology (NIAB) are advancing efforts to produce a novel vaccine for ‘Leptospirosis’—a significant illness impacting both humans and animals, attributable to bacteria known as ‘Leptospira’, which consists of over 300 distinct strains. This zoonotic disease has emerged as an increasing public health threat, exacerbated by climate change and global warming factors.
The Declining Efficacy of Antibiotics
Around one million human cases of leptospirosis arise each year, contributing to approximately 60,000 fatalities. Although there is an assortment of antibiotics available for treatment, their efficacy diminishes when bacteria invade critical organs—often due to late diagnosis—as noted by scientists from the Institute affiliated with the Department of Biotechnology.
Vaccination presents a proven and economically viable preventive strategy against this ailment; however, existing killed vaccines offer only temporary immunity limited to certain strains and do not prevent bacterial excretion in urine.
Current Vaccine Landscape
Presently, vaccines exist exclusively for animals but fail to cover all bacterial strains involved; additionally, there are no approved human vaccines available. Current veterinary vaccines may induce strong cross-protective effects but lack sterilizing immunity or lasting protective responses.
Focus on Next-Generation Vaccination by NIAB
The NIAB research team led by Syed Faisal is committed to developing next-generation vaccines that target multiple Leptospira strains. They have successfully characterized Lipopolysaccharide (LPS), which serves as a crucial protective antigen defining strain specificity according to Director G. Taru Sharma.
This research team has also found that initial immune reactions against LPS can determine whether an individual will face mild symptoms or severe infections characterized by multi-organ dysfunction. Notably, they identified that Lipid A—a component derived from LPS—is less toxic yet capable of enhancing the immune response thereby increasing vaccine efficacy—a significant breakthrough toward innovative vaccine development.
The study recognized various antigens like Leptospira immunoglobulin-like proteins named ‘LigA and LigB’ as promising candidates for subunit vaccination; these require effective adjuvants for optimal performance. Trials conducted using mice and hamsters revealed that combining these proteins with alum alongside Leptospira Lipid A outperformed other combinations in eliciting robust cellular immune responses while providing sterilizing immunity against leptospirosis.
“This comprehensive research supported by the Department of Science & Technology (DST) emphasizes the adjuvant properties inherent in Leptospira Lipid A while paving promising paths towards LPS-based vaccinations aimed at combating this serious zoonotic affliction,” stated Dr. Faisal. Moreover, he noted that potent adjuvants invigorate innate immune mechanisms leading towards sustained antigen-specific protective responses.” This pivotal work has been documented in ‘Open Biology and Vaccines’, a renowned international journal.
Published – October 29, 2024 01:50 pm IST